Lewis Structure
Moderators: Chem_Mod, Chem_Admin
-
Tiffany Cao 1D
- Posts: 94
- Joined: Fri Sep 29, 2017 7:03 am
- Been upvoted: 1 time
Lewis Structure
For compounds such as ClONO2, how will we be able to determine which element is in the middle and the overall organization of the Lewis structure?
-
Alex Kashou
- Posts: 38
- Joined: Fri Sep 29, 2017 7:07 am
Re: Lewis Structure
When looking at a molecule that is complex like this, you should follow the way in which the elements are ordered. So ClONO2 would have cl on the far left connected to an O, then with an NO2 ion connected to that O as well. This is a general rule I learned in AP chem because we have not learned how to determine which is central atom of more complex molecules such as these.
Rule of thumb though the one with highest ionization energy usually goes in the center for simpler molecules.
We will not need to know anything much more complex according to my TA as well.
Hope this helped!
Rule of thumb though the one with highest ionization energy usually goes in the center for simpler molecules.
We will not need to know anything much more complex according to my TA as well.
Hope this helped!
-
Sean Monji 2B
- Posts: 66
- Joined: Fri Sep 29, 2017 7:06 am
Re: Lewis Structure
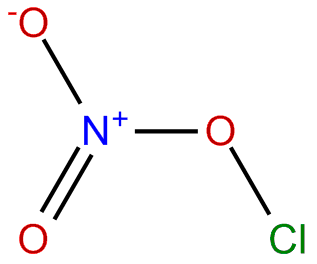
Chlorine nitrate right? We'll probably learn this later, but since it is written as ClONO2 in this case instead of CLNO3 to make it easier for us, you can think of it as CLO bonding with NO2.
CLO bonds to create a full octet plus 2 electrons for chlorine and a lone electron for oxygen (explained in posts about chlorine monoxide).
NO2 creates a bent structure due to the lone electron in the nitrogen while the oxygen have a full octet (since oxygen has a higher electron affinity than nitrogen).
/NO2_Dot-56a12a2c3df78cf772680359.png)
The lone electron of the oxygen in CLO and lone electron of NO2 bond together to fill the octet of the oxygen in CLO and nitrogen of NO2, forming CLN03 (the lone electrons pair to create a single bond).
The name, chlorine nitrate, also sort of gives away that the nitrogen has 3 oxygen bonded to it, but that wasn't given.
-
Helen Shi 1J
- Posts: 78
- Joined: Sat Jul 22, 2017 3:00 am
-
Sean Monji 2B
- Posts: 66
- Joined: Fri Sep 29, 2017 7:06 am
Re: Lewis Structure
For now, I think it’s just a general rule for making Lewis dot structures. The reason is most likely due to the lower ionization atom more easily gives away or share electrons. They also usually have more unpaired electrons (seen in the electron config) that can be paired for bonding. (N has 3 while Cl has 1)
Who is online
Users browsing this forum: No registered users and 9 guests

