Determining when to use double bonds [ENDORSED]
Moderators: Chem_Mod, Chem_Admin
Determining when to use double bonds
Hello,
I was just a bit confused during the lecture yesterday about how we know when to replace a single bond that we have drawn with a double bond?
Thank you
I was just a bit confused during the lecture yesterday about how we know when to replace a single bond that we have drawn with a double bond?
Thank you
-
Rhea Desai 1A
- Posts: 112
- Joined: Fri Sep 24, 2021 6:32 am
- Been upvoted: 1 time
Re: Determining when to use double bonds
We first count the number of valance electrons we have in total. If we draw single bonds and we have used too many electrons, then its likely that we need to change a single bond into a double bond to lower the amount of electrons used.
-
Jacqueline Duong 1D
- Posts: 51
- Joined: Fri Sep 24, 2021 6:33 am
Re: Determining when to use double bonds
On a more technical level, I believe it has to do with formal charge and finding the preferred structure of the molecule that is most stable. Like Rhea mentioned, it has to do with how many valence electrons we have available to see whether or not we can use lone pairs or shared double (or triple) bonds to create the lewis structure.
-
Cassidy Chiong 2J
- Posts: 118
- Joined: Fri Sep 24, 2021 6:26 am
Re: Determining when to use double bonds
Double bonds can be included when the use of single bonds results in the molecule having too many electrons, or when it results in a more stable molecule. However, it is also important to note the formal charge of the atoms within a molecule, since this tells which type of bond (i.e. single, double, triple, etc.) is more favorable by resulting in a more stable molecule. Formal charges of 0 are most stable.
-
Allison Peng 1D
- Posts: 152
- Joined: Fri Sep 24, 2021 6:19 am
Re: Determining when to use double bonds
A typical case where you;d use a double bond between atoms as opposed to a single one is when there are too many electrons in the structure you've drawn with only one bond. In this case, sharing more electrons will lead to a lower number of total electrons. Additionally, for the case of sulfate, we know each oxygen has 8 electrons to lead to the total number of 32 electrons, so single bonds technically work, but looking at formal charge and considering expanded valence, the addition of double bonds will make the molecule more stable.
-
Crystal Ma 2J
- Posts: 100
- Joined: Fri Sep 24, 2021 7:04 am
Re: Determining when to use double bonds
One example would be that when using single bonds, we have used up too many electrons (more than the number of valence electrons we've counted).
-
Andrew Nguyen 1E
- Posts: 101
- Joined: Fri Sep 24, 2021 6:34 am
- Been upvoted: 1 time
Re: Determining when to use double bonds
We would use double bonds instead of single bonds if when using single bonds, we run out of valence electrons or if the formal charge of the atoms bonded is lower when a double bond is used.
In class, for the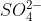 ion professor replaced two single bonds around the sulfur atom because in doing so, he lowered formal charges to 0, making the Lewis structure more stable.
ion professor replaced two single bonds around the sulfur atom because in doing so, he lowered formal charges to 0, making the Lewis structure more stable.
In class, for the
-
Helen Ringley 2E
- Posts: 129
- Joined: Fri Sep 24, 2021 7:36 am
Re: Determining when to use double bonds
so when you are calculating formal charges, double bonds get divided by 2 but lone pairs are counted as whole electrons. This means that if we have a formal charge that we want to decrease, aka make the atom more stable, we can turn lone pairs into double bonds.
-
Triston Dinh 1D
- Posts: 101
- Joined: Fri Sep 24, 2021 6:03 am
Re: Determining when to use double bonds
We use double bonds if there are not enough electrons left over to fill the octet of every element in the Lewis structure. Creating a double bond will allow two elements to share an extra two electrons which makes up for the lack of electrons.
-
Talia Tam 3L
- Posts: 107
- Joined: Fri Sep 24, 2021 5:38 am
Re: Determining when to use double bonds [ENDORSED]
One way to determine when to use double bonds is when using only single bonds result in too many valence electrons.
-
Helen Ringley 2E
- Posts: 129
- Joined: Fri Sep 24, 2021 7:36 am
Re: Determining when to use double bonds
It's a really effective way to reduce the number of electrons used in a lewis structure because they can fulfill the octet of two atoms but only uses two electrons.
-
Alejandra Hernandez 2A
- Posts: 98
- Joined: Fri Sep 24, 2021 7:04 am
Re: Determining when to use double bonds
Double bonds are used when there are not enough electrons to fulfill an atom's octet using single bonds, however, they can also be used when creating resonance structures in order to give atoms the most desirable formal charge. Hope this helped :)
-
oliviahelou
- Posts: 103
- Joined: Fri Sep 24, 2021 6:36 am
Re: Determining when to use double bonds
When using single bonds results in too many valence electrons, it's always good to switch to double bonds.
-
ElizabethKarlin2E
- Posts: 49
- Joined: Fri Sep 24, 2021 6:30 am
Re: Determining when to use double bonds
I think you switch to double bonds when you don't have enough electrons to form the octet with just a single bond.
-
Ivy Vo Dis 1C
- Posts: 100
- Joined: Fri Sep 24, 2021 5:48 am
Re: Determining when to use double bonds
Double bonds are used in order to fill the outer shell of two elements while using fewer electrons. A single bond would act as two valence electrons for each atom involved. Meanwhile, a double bond would act as four valence electrons for each atom involved. We should use these double bonds when we need more electrons to fill up the outer shells of atoms, but have already run out.
Re: Determining when to use double bonds
Before you make a Lewis structure, you should count all of the valence e- the various elements within the molecule have. Then, draw the lewis structure and add unpaired e- to atoms that do not have an octet. If there are too many e- needed to satisfy octets that are available, use a double/triple bond.
-
HannahArabi14a
- Posts: 37
- Joined: Fri Sep 24, 2021 5:35 am
Re: Determining when to use double bonds
Hey! I'm going to give you an example of a chemical compound that would need a double bond: NO2-.
First you have to calculate the amount of electrons in each element. In nitrogen you will find there are 5e- and in O there is 6e-, however since there are 2 oxygen present it would be 12e-. Adding the electrons from nitrogen and oxygen I get a total of 17e- and I add the ionic charge from NO2-, for 18e-.
When drawing a lewis structure you will find that one of the oxygen would require an octet by a double bond with nitrogen, so that the octet requirement is satisfied for both nitrogen and oxygen.
Hope this helps!
First you have to calculate the amount of electrons in each element. In nitrogen you will find there are 5e- and in O there is 6e-, however since there are 2 oxygen present it would be 12e-. Adding the electrons from nitrogen and oxygen I get a total of 17e- and I add the ionic charge from NO2-, for 18e-.
When drawing a lewis structure you will find that one of the oxygen would require an octet by a double bond with nitrogen, so that the octet requirement is satisfied for both nitrogen and oxygen.
Hope this helps!
-
Jennifer Kainth 3L
- Posts: 103
- Joined: Fri Sep 24, 2021 6:11 am
Re: Determining when to use double bonds
To begin, determine the total number of valance electrons.
When the usage of single bonds results in an excess of electrons in the molecule, a double bond can be utilized to decrease the number of electrons used.
When the usage of single bonds results in an excess of electrons in the molecule, a double bond can be utilized to decrease the number of electrons used.
-
Mahima Manoj 1F
- Posts: 50
- Joined: Fri Sep 24, 2021 6:09 am
Re: Determining when to use double bonds
When you count the total valence electrons of the molecule and the single bond valence electrons are greater than the total valence electrons of the structure that's an indication you need to use double bonds.
-
madelyn kelly 1I
- Posts: 53
- Joined: Fri Sep 24, 2021 6:24 am
Re: Determining when to use double bonds
Double bonds can be used when there are not enough electrons to satisfy an element's octet through single bonds. Instead of having not enough bonds, you can replace the single bond with a double to satisfy the octet without using too many electrons. In addition, double bonds can be used in resonance structure because sometimes they may lead to a lower force charge, leading to a molecule becoming more stable.
Who is online
Users browsing this forum: No registered users and 10 guests

