Finding all Resonance Structures
Moderators: Chem_Mod, Chem_Admin
-
rosemarywang4i
- Posts: 34
- Joined: Fri Sep 28, 2018 12:28 am
Finding all Resonance Structures
Many of the textbook problems ask to find resonance structures for compounds— are there any tricks to how we can find ALL of the resonance structures? So far I've just been drawing out a lot as trial and error but I was wondering if there's any other ways to go about this.
-
Shash Khemka 1K
- Posts: 38
- Joined: Fri Sep 28, 2018 12:18 am
Re: Finding all Resonance Structures
I don't think there are any "tricks" for resonance structures. It's pretty much just trial and error while making sure formal charges fit the structure. The only "trick" I can think of is when there are double bonds to similar elements that can be exchanged with each other such as:
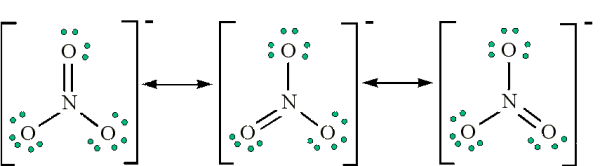
- Attachments
-
- Resonance.GIF (8.75 KiB) Viewed 500 times
-
Peichung Chou 1A
- Posts: 30
- Joined: Fri Sep 28, 2018 12:21 am
Re: Finding all Resonance Structures
You should be able to determine if something is a resonant structure from formal charge tests, as a resonant structure will contain one or more bonds of multiple bond order. However, otherwise there is no trick to determine whether something is a resonant structure.
Re: Finding all Resonance Structures
I don't think there are certain "tricks" in drawing resonance structures. The best way would be to look at the formal charges and making sure they are as close to zero (or add up to the ion charge) If the structure consists of different types of bonds and you notice that the different types of bonds (for example a double bond can be draws on either oxygen atom) then we could draw two structures showing that it doesn't really matter which oxygen atom the double bond is connected to, and that the most stable structure is an average of the bond lengths.
-
Jack Mitchell 3J
- Posts: 24
- Joined: Fri Sep 28, 2018 12:24 am
Re: Finding all Resonance Structures
I would say the only "trick" is seeing if there are different places where the same bond could possibly be placed without the formal charge being changed at all, but this really isn't a trick.
Return to “Resonance Structures”
Who is online
Users browsing this forum: No registered users and 6 guests

