Hello,
I was wondering if anyone knows how to find oxidation numbers. It would be really helpful to know the steps into finding the answers. If someone can explain, that would be great. Thank you.
Oxidation Numbers
Moderators: Chem_Mod, Chem_Admin
-
Maximillian Ryan 2K
- Posts: 76
- Joined: Fri Sep 29, 2023 12:22 pm
Re: Oxidation Numbers
The oxidation number of an atom is, in effect, the charge that atom has on it. For example, in iron (II) oxide, the oxidation number of iron is 2 because it has lost two electrons to the oxygen atom in this ionic compound.
-
Christian_Lee_2K
- Posts: 174
- Joined: Fri Sep 29, 2023 12:02 pm
Re: Oxidation Numbers
Hi there!
To find oxidation state for pure elements, this chart that my TA gave me helps!
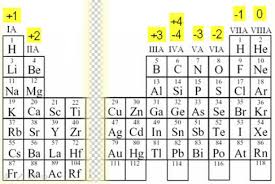
For compounds, you can draw the Lewis structure to find the charge and that'll be the oxidative state. I hope this helps
Other compounds/molecules are also provided to us in this PDF.
To find oxidation state for pure elements, this chart that my TA gave me helps!
For compounds, you can draw the Lewis structure to find the charge and that'll be the oxidative state. I hope this helps
Other compounds/molecules are also provided to us in this PDF.
-
Valeria Morales
- Posts: 39
- Joined: Mon Jan 09, 2023 2:11 am
Re: Oxidation Numbers
Hi,
If you remember the ionic trends of the periodic table, we know that the groups have specific charges to them. For example group 1 has a +1 charge G2 +2 G3 +3. If we take SO4 -2 for example. If we are solving for the oxidation number of S, we can look at the periodic trend and we know that O has a -2 charge and there are 4 oxygen. We know our overall charge is -2 as well. If we plug it into an equation were X is our unknown oxidation number S we have:
X + -2(4) =-2
X - 8= -2
X = +6
Now we know that our oxidation number of S is 6 in our molecule of SO4 -2.
Keep in mind that oxidation number and ionic charge are not the same thing.
If you remember the ionic trends of the periodic table, we know that the groups have specific charges to them. For example group 1 has a +1 charge G2 +2 G3 +3. If we take SO4 -2 for example. If we are solving for the oxidation number of S, we can look at the periodic trend and we know that O has a -2 charge and there are 4 oxygen. We know our overall charge is -2 as well. If we plug it into an equation were X is our unknown oxidation number S we have:
X + -2(4) =-2
X - 8= -2
X = +6
Now we know that our oxidation number of S is 6 in our molecule of SO4 -2.
Keep in mind that oxidation number and ionic charge are not the same thing.
Return to “Formal Charge and Oxidation Numbers”
Who is online
Users browsing this forum: No registered users and 6 guests

