Hi!
How do I know what would be the most stable Lewis Structure in terms of formal charge? I know that we should maximize the number of atoms that have a 0 formal charge, but how do I know when I should add a double bond or something to reduce the FC?
Thanks!
Determining Best Lewis Structure
Moderators: Chem_Mod, Chem_Admin
-
Cadence Chang
- Posts: 135
- Joined: Fri Sep 24, 2021 6:05 am
- Been upvoted: 1 time
-
Chem_Mod
- Posts: 23858
- Joined: Thu Aug 04, 2011 1:53 pm
- Has upvoted: 1253 times
Re: Determining Best Lewis Structure
You will add a double bond when doing so reduced the formal charges of one or more atoms to zero. You can play around with different double bonding to different atoms and see which produces the most minimal formal charge. Additionally, if there is a negative formal charge, it should be on the most electronegative atom!
Re: Determining Best Lewis Structure
Basically you just have to make the formal charge of the most atoms in the molecule close to zero.
-
Aneesha_Nema_3C
- Posts: 96
- Joined: Fri Sep 24, 2021 5:22 am
Re: Determining Best Lewis Structure
Like the previous people said, you can just play around with double bonds to see if they are able to bring the formal charge of individual atoms closer to 0. There are no strict rules you have to follow other than the octet rule guidelines (unless exceptions apply to the atoms/molecules).
-
Robert Nguyen 14B-3E
- Posts: 113
- Joined: Fri Sep 24, 2021 6:23 am
- Been upvoted: 1 time
Re: Determining Best Lewis Structure
A tip to figuring out which bonds to change would be looking at which bonds are connected to charges that could be lowered
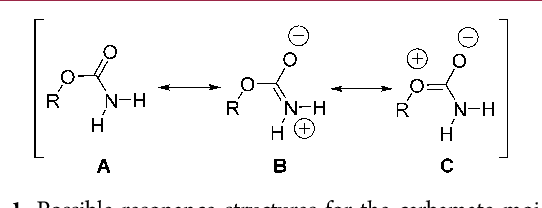
In the above image for example, we can see in the middle structure that the central carbon has two bonds connected to an atoms with formal charges: one oxygen and one nitrogen (If it's hard to tell from the model, this is because the only single bond of the oxygen forces it to have 6 other electrons, totaling 7, which is one more negative than its normal value (6-), while the nitrogen's double bond to the carbon plus its two hydrogen atom bonds make it only have a formal charge of 4, which is one more positive than its normal value (7-)). As we can see in the left structure, this can be changed by changing both bonds affected to them: turning the oxygen bond to a double bond removes the need for 2 of the other lone pair electrons, giving it a formal charge of 6 (2 bonds + 2 pairs), and the nitrogen's single bond would now force it to need another pair of electrons, taking its formal charge to 5 (1 carbon bond + 2 hydrogen atom bonds + 1 pair), which for both is the standard value.

In the above image for example, we can see in the middle structure that the central carbon has two bonds connected to an atoms with formal charges: one oxygen and one nitrogen (If it's hard to tell from the model, this is because the only single bond of the oxygen forces it to have 6 other electrons, totaling 7, which is one more negative than its normal value (6-), while the nitrogen's double bond to the carbon plus its two hydrogen atom bonds make it only have a formal charge of 4, which is one more positive than its normal value (7-)). As we can see in the left structure, this can be changed by changing both bonds affected to them: turning the oxygen bond to a double bond removes the need for 2 of the other lone pair electrons, giving it a formal charge of 6 (2 bonds + 2 pairs), and the nitrogen's single bond would now force it to need another pair of electrons, taking its formal charge to 5 (1 carbon bond + 2 hydrogen atom bonds + 1 pair), which for both is the standard value.
Return to “Formal Charge and Oxidation Numbers”
Who is online
Users browsing this forum: No registered users and 2 guests

