Hello! The last part of this problem asked if AsO4 -3 was polar or nonpolar. I initially thought it was polar, however the solution said:
"Although the bonds here are polar, the molecule is not due to resonance and the symmetrical shape of the molecule."
Could someone please clarify this for me? Thank you!
Sapling Q.20
Moderators: Chem_Mod, Chem_Admin
-
shevanti_kumar_1E
- Posts: 116
- Joined: Wed Sep 30, 2020 9:40 pm
- Been upvoted: 1 time
Re: Sapling Q.20
The molecule is not polar because the dipoles of the polar bonds (As-O) cancel out. The shape of AsO4- is tetrahedral.Since all the exterior atoms are the same (O), the dipoles of the polar bonds cancel out and the molecule overall is not a polar molecule.
-
Sandy Lin 1L
- Posts: 102
- Joined: Wed Sep 30, 2020 9:48 pm
Re: Sapling Q.20
You can tell whether a molecule is polar or not by looking at the lewis structure. Since you know that the molecular shape is tetrahedral and that all the atoms surrounding the central atom (As) are all the same, the molecule overall is nonpolar because the dipoles in the polar As and O bonds will cancel each other out.
-
Michael Iter 2F
- Posts: 101
- Joined: Wed Sep 30, 2020 9:48 pm
Re: Sapling Q.20
Polarity of a molecule depends on the polarity of its bonds as well as its symmetry. Since the molecule in question is symmetrical, there is no net dipole moment and thus it is nonpolar
-
AndrewNguyen_2H
- Posts: 100
- Joined: Wed Sep 30, 2020 9:59 pm
Re: Sapling Q.20
There can exist polar dipoles between individual atoms, but the overall molecule would still be nonpolar if these dipoles are equal in magnitude and opposite in direction. "As" is surrounded by oxygens in a tetrahedral shape. The oxygens are all the same so it is nonpolar. There is a resonance here with the double bond, but remember resonance is merely multiple descriptions that contribute to an actual molecule, so each bond will still be equal.
-
Jonathan Banh 1G
- Posts: 60
- Joined: Wed Sep 30, 2020 9:33 pm
Re: Sapling Q.20
Like Andrew said, the ion's structure will be a resonance hybrid in the end so all of its bonds will be relatively the same. To find out the polarity of 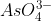 , it is more important to focus on the molecular geometry. We can easily recognize that each of the bonds in the molecule are polar since oxygen is much more electronegative than arsenic. However, because of the molecule's tetrahedral shape, it causes the dipole moments (polar bonds) to cancel each other out. This is because all of the atoms are the same (oxygen) and thus, the shape is symmetrical. This shows that
, it is more important to focus on the molecular geometry. We can easily recognize that each of the bonds in the molecule are polar since oxygen is much more electronegative than arsenic. However, because of the molecule's tetrahedral shape, it causes the dipole moments (polar bonds) to cancel each other out. This is because all of the atoms are the same (oxygen) and thus, the shape is symmetrical. This shows that 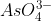 is a nonpolar molecule despite having polar bonds within its structure. In understanding the polarity of molecules, it is important to not rely on the polarity of bonds as the sole determinant, but instead use it in tandem with information about the molecular geometry at hand.
is a nonpolar molecule despite having polar bonds within its structure. In understanding the polarity of molecules, it is important to not rely on the polarity of bonds as the sole determinant, but instead use it in tandem with information about the molecular geometry at hand.
-
Veeda Khan 2E
- Posts: 52
- Joined: Wed Sep 30, 2020 10:00 pm
-
Brian_Wu_3B
- Posts: 103
- Joined: Wed Sep 30, 2020 9:41 pm
Re: Sapling Q.20
Oh. I thought it was polar because it had a charge. Does this not make the entire molecule polar?
-
Janys Li - 1L
- Posts: 52
- Joined: Fri Sep 24, 2021 5:13 am
Re: Sapling Q.20
Hi!
I was also wondering about this question and did not understand the explanation. But I soon realized that all the atoms around the central atom are the same, all oxygen, which means that the double bond will have resonance, which means that some electrons would be universally shared between the bonds, making it a nonpolar molecule.
I was also wondering about this question and did not understand the explanation. But I soon realized that all the atoms around the central atom are the same, all oxygen, which means that the double bond will have resonance, which means that some electrons would be universally shared between the bonds, making it a nonpolar molecule.
Return to “Polarisability of Anions, The Polarizing Power of Cations”
Who is online
Users browsing this forum: No registered users and 9 guests

