Clarification on Dipole Moment
Moderators: Chem_Mod, Chem_Admin
-
Malik Oda 1F
- Posts: 51
- Joined: Thu Jul 25, 2019 12:15 am
Clarification on Dipole Moment
Can someone please clarify the concept of dipole moment and how to calculate it?
-
SGonzales_3L
- Posts: 55
- Joined: Thu Jul 11, 2019 12:17 am
Re: Clarification on Dipole Moment
A dipole moment measures the partial charges on atoms in a molecule. It shows which has a more negative charge due to a higher affinity for electrons and which has a more positive charge. This shows how polar a molecule will be and also shows how covalent bonds may have ionic character.
You can calculate the dipole moment using the charge and distance between the atoms.
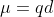 where
where 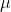 is the dipole moment, q is the charge, and d is the distance between the atoms.
is the dipole moment, q is the charge, and d is the distance between the atoms.
You can calculate the dipole moment using the charge and distance between the atoms.
-
WesleyWu_1C
- Posts: 117
- Joined: Thu Jul 25, 2019 12:16 am
Re: Clarification on Dipole Moment
The easier way to calculate which atom has the partial positive charge and which atom has the partial negative charge is by looking at the eletronegativity for each atom. If the atom has a higher electronegativity then the other atom, then it will have the partial negative charge.
-
Lauren Sanchez 3D
- Posts: 52
- Joined: Sat Aug 24, 2019 12:17 am
Who is online
Users browsing this forum: No registered users and 5 guests

