Dipole Moments Cancelling out
Moderators: Chem_Mod, Chem_Admin
-
Angel Gutierrez 2E
- Posts: 51
- Joined: Wed Sep 30, 2020 9:49 pm
Dipole Moments Cancelling out
In Dr. Lavelle's Friday (Nov. 20) lecture, he explains that a polar molecule does not have dipole moments that cancel out. In cis-dichloretene, the dipole moments point away from each other. In a non polar molecule, the dipole moments, still point away from each other but they are still considered to cancel out. WHy is that? How can you determine if the dipole moments cancel out?
-
Chloe Little 3K
- Posts: 102
- Joined: Wed Sep 30, 2020 10:02 pm
- Been upvoted: 1 time
Re: Dipole Moments Cancelling out
For the dipoles to cancel out, they must point in opposite directions. In cis-dichloroethene, the dipoles are pointing in different directions but they are not completely opposite. In other words, the directions of the dipoles must be 180 degrees apart for them to cancel out.
-
George Cazares 1E
- Posts: 51
- Joined: Wed Sep 30, 2020 9:39 pm
- Been upvoted: 1 time
Re: Dipole Moments Cancelling out
In cis-dichloroethene, the dipoles are in the same general direction, so there is a net dipole to one side, which makes it polar. However, in trans-dichloroethene, the dipole moments are on opposite sides, which cancel, making it nonpolar.
-
Katie Nye 2F
- Posts: 100
- Joined: Wed Sep 30, 2020 9:55 pm
Re: Dipole Moments Cancelling out
I always look to see if there's a way that i can fold the molecule to find symmetry. So if you were to cut the molecule in half and fold it, you would have two pieces that are identical which means the dipole moments cancel out.
-
Sabine Salvucci 2E
- Posts: 100
- Joined: Wed Sep 30, 2020 9:42 pm
- Been upvoted: 1 time
Re: Dipole Moments Cancelling out
Hello! For a molecule with dipole moments to be non polar, the dipoles have to point in opposite directions. In cis-dichloroethene the dipoles point in different directions, but since the Cl atoms are on the same side of the molecule, they aren't completely opposite and wouldn't cancel each other out. Hope this helps!
-
Sahiti Annadata 3D
- Posts: 103
- Joined: Wed Sep 30, 2020 10:01 pm
Re: Dipole Moments Cancelling out
For the dipole moments to cancel out, and the molecule to be nonpolar the molecule's dipole moments need to be symmetrical. The way you can tell if a molecule is symmetrical is through VSEPR theory.
-
Eliot Kagan 2G
- Posts: 100
- Joined: Wed Sep 30, 2020 10:08 pm
Re: Dipole Moments Cancelling out
I think looking at the forces in terms of vectors may help. Note that the example below works because the atoms surrounding the dipole moments are exactly the same, thus having the same forces acted upon it (just in a different direction).
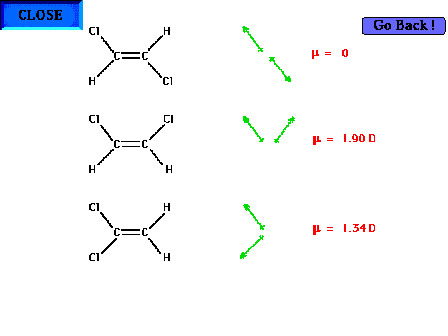
-
Tanner Bartyczak 1K
- Posts: 100
- Joined: Wed Sep 30, 2020 9:51 pm
Re: Dipole Moments Cancelling out
I agree with what the other comments have said. The diagram is really helpful in visualizing this interaction.
-
Malakai Espinosa 3E
- Posts: 103
- Joined: Wed Sep 30, 2020 9:50 pm
- Been upvoted: 1 time
Re: Dipole Moments Cancelling out
The dipoles have to cancel in a linear manner, kind of like tug of war where both sides are equal in strength. If you tried to do tug of war when both sides are not completely opposite from one another, it wouldn't really work. Hope this analogy helps :)
-
Nicole Bruno Dis 1B
- Posts: 51
- Joined: Wed Sep 30, 2020 9:36 pm
Re: Dipole Moments Cancelling out
If the polar bonds are arranged symmetrically, this means the positive and negative charges coincide and thus no dipole moment occurs. The bond dipoles essentially cancel and the molecule becomes nonpolar.
-
isha dis3d
- Posts: 106
- Joined: Wed Sep 30, 2020 9:47 pm
Re: Dipole Moments Cancelling out
It is also important to note that different electronegativities between elements indicate different "vector" lengths when drawing the dipole moments out. This could result in a net vector or dipole in one direction.
Who is online
Users browsing this forum: No registered users and 8 guests

