Bond length in double vs single [ENDORSED]
Moderators: Chem_Mod, Chem_Admin
-
August Blum Dis 3D
- Posts: 54
- Joined: Fri Sep 24, 2021 5:30 am
Bond length in double vs single
Why is it that double bonds are shorter than single bonds? Is it due to energy?
Re: Bond length in double vs single
accoring to google "Double bonds are shorter than single bonds because double bonds are stronger and therefore pull the electrons closer together in the two elements"
Re: Bond length in double vs single
double bonds are shorter because more electrons are being shared between two atoms. therefore, they are more attracted to one another and pulled in more leading to a shorter bond length compared to a single bond.
-
Natalie Gibson 1c
- Posts: 105
- Joined: Fri Sep 24, 2021 7:22 am
Re: Bond length in double vs single
When thinking of bonds length, this is directly proportional to bond strength. A double bond is going to be stronger than a single bond, which therefore pulls the electrons closer together between the two atoms. A single bond would not be as strong as the double bond, hence the longer bond length. Using this same reasoning we can also determine that a triple bond would be stronger, and shorter, than a double or single bond.
-
Jonathan Alterman 1C
- Posts: 110
- Joined: Fri Sep 24, 2021 6:35 am
Re: Bond length in double vs single
Hi! Double bonds are shorter than single bonds because they are stronger than single bonds. As a result, more energy is needed to break a double bond compared to a single bond. Furthermore, a triple bond would be even stronger and shorter than a double bond, so even more energy is required to break it.
-
Hanyi Jia 3B
- Posts: 118
- Joined: Fri Sep 24, 2021 6:08 am
- Been upvoted: 1 time
Re: Bond length in double vs single
A double bond involves a sigma bond and a pi bond. A pi bond is the side-by-side overlapping between two p orbitals which has a much shorter distance than the sigma bond is a single bond (which is head to head). See the figure below. The pi bond makes the bond length of the double bond short.
And of course, the bond strength is stronger for the double bond is stronger than a single bond, but I won't say that as a "reason" to explain the length. Strength is more like the result.
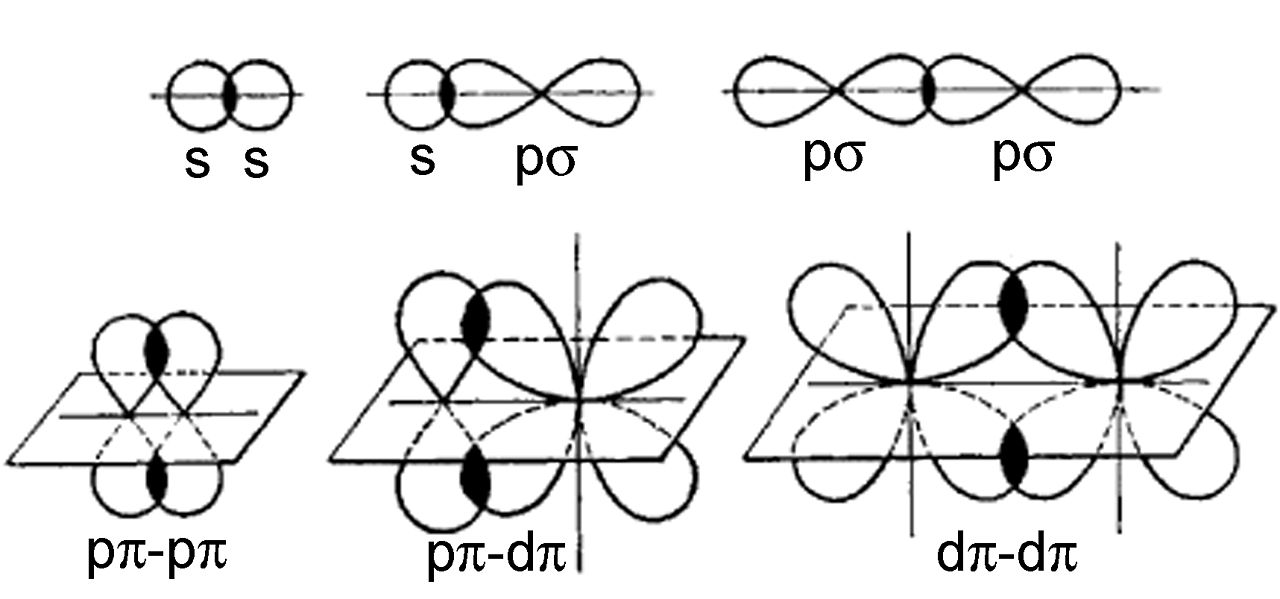
I think we will be talking about it this week.
And of course, the bond strength is stronger for the double bond is stronger than a single bond, but I won't say that as a "reason" to explain the length. Strength is more like the result.

I think we will be talking about it this week.
-
Kaitlyn_Urquilla_1I
- Posts: 102
- Joined: Fri Sep 24, 2021 7:15 am
Re: Bond length in double vs single
Yes, it is. A double bond is shorter than a single bond because more electrons are being shared and that means that it would take more energy to separate the atoms compared to a single bond. The bond length corresponds to how much energy it takes to break that bond, the shorter the bond length the more energy it takes to break that bond.
-
Michael 1G
- Posts: 101
- Joined: Fri Sep 24, 2021 6:16 am
- Been upvoted: 1 time
Re: Bond length in double vs single
A double bond is shorter than a single bond because they are stronger and therefore pull electrons tighter together. Since single bonds are lower in strength they will not pull as hard, making them longer.
-
Shania Garrison Discussion 3E
- Posts: 149
- Joined: Wed Feb 17, 2021 12:24 am
Re: Bond length in double vs single
Lavelle said that double bonds are stronger and therfore shorter, while single bonds are weaker and therefore longer.
-
Charlie Gravereaux
- Posts: 101
- Joined: Fri Sep 24, 2021 5:51 am
Re: Bond length in double vs single
Double bonds are sharing more electrons between the atoms, meaning that there will be a greater/stronger attraction between the two atoms, pulling them in closer toward each other. Because the strength of the double is greater, the length is therefore shorter compared to a single bond, which shares less electrons and therefore the attraction between atoms is weaker.
-
SerenaSabedra
- Posts: 102
- Joined: Fri Sep 24, 2021 7:05 am
Re: Bond length in double vs single
Double as well as triple bonds are able to pull electrons in two different atoms closer together than a single bond, which then results in a bond that is harder to break because these electrons are closer to the atoms.
-
asfakhan_2H
- Posts: 106
- Joined: Wed Nov 18, 2020 12:25 am
Re: Bond length in double vs single
So double bonds are shorter because they have a higher attraction causing them to be tighter and pulled in more closely. The single bonds aren't as strong which causes them to be longer
-
Joseph Lee
- Posts: 100
- Joined: Fri Sep 24, 2021 6:35 am
Re: Bond length in double vs single
Hello! We covered this in lecture today, but shorter bond lengths mean that there is a stronger attraction, while longer ones usually tend to mean that they are weaker. Also double bonds are rarely broken for reactions, while longer and single ones are. And don't forget, electron electron repulsion in single bond pairs usually play a role as well.
-
Madison Nguyen 3L
- Posts: 103
- Joined: Fri Sep 24, 2021 7:03 am
Re: Bond length in double vs single
A double bond is stronger than a single bond by pulling more electrons closer together between the two atoms (comparatively to a single bond as well). Thus, because the stronger a bond is, the shorter its length becomes, then a double bond would be shorter than a single bond.
-
Fiona H 2E
- Posts: 106
- Joined: Fri Sep 24, 2021 5:26 am
Re: Bond length in double vs single
Double bonds are shorter than single bonds because double bonds are stronger and therefore pull electrons closer together in the two elements which decreases the overall length of the bond.
Re: Bond length in double vs single
Double bonds are shorter than single bonds because they are stronger bonds. The electrons involved in double bonds are pulled together with a stronger force when compared to a single bond. When pulled with a stronger force the bond becomes shorter.
-
Kaira Shibata 1E
- Posts: 105
- Joined: Fri Sep 24, 2021 5:26 am
- Been upvoted: 1 time
Re: Bond length in double vs single
Double bonds are shorter and therefore have less energy. Less energy implies greater stability and greater strength.
-
Elizabeth Kim 2K
- Posts: 50
- Joined: Fri Sep 24, 2021 5:11 am
Re: Bond length in double vs single
Hi! Double bonds are shorter than single bonds because they have a stronger pull on the electrons, so they pull electrons in closer, which results in a shorter bond length. Hope this helps!
-
Ginny Ghang 1B
- Posts: 102
- Joined: Fri Sep 24, 2021 7:22 am
Re: Bond length in double vs single
Since double bonds are stronger, they pull the atoms closer together than the single bond does. Stronger bonds hold the atoms more closer together.
-
Katherine Li 1A
- Posts: 110
- Joined: Fri Sep 24, 2021 6:38 am
Re: Bond length in double vs single
That's because a double bond is stronger and shares more e-, so the atoms will pull the e- in closer. Think of it as holding one hand versus holding two hands with someone--you'll have to stand closer to them if you're holding two hands.
-
Jahnavi Srinivas 2H
- Posts: 50
- Joined: Fri Sep 24, 2021 6:02 am
Re: Bond length in double vs single
Double bonds pull electrons with a greater force than single bonds. This greater force also means the electrons are pulled closer, so more strength in a double bond correlates to a shorter bond. A single bond is weaker and therefore longer.
-
Martha Avila 1I
- Posts: 100
- Joined: Fri Sep 24, 2021 6:21 am
- Been upvoted: 1 time
Re: Bond length in double vs single
Hello! Double bonds are shorter than single bonds because these double bonds are stronger which means that they are closer and pulling the electrons strongly. We generally know that if a bond is weak it is going to be longer and more easy to break. A bond that is strong is much smaller and harder to break. Hope this helps.
-
Sarah Hong 2K
- Posts: 102
- Joined: Fri Sep 24, 2021 5:48 am
Re: Bond length in double vs single
Double bonds are stronger and shorter than single bonds because double bonds have 2 pairs of bonding electrons whereas single bonds only have 1 pair of bonding electrons. The two pairs in double bonds pull the atoms closer together creating shorter and stronger bond lengths.
-
Genelle Marcelino-Searles 2G
- Posts: 102
- Joined: Sun Sep 26, 2021 5:00 am
Re: Bond length in double vs single
Double bonds have a shorter bond length than single bonds because the double bonded atoms are pulled together with more strength than the single bonds.
-
bree_wray_14a
- Posts: 50
- Joined: Fri Sep 24, 2021 6:33 am
Re: Bond length in double vs single
August Blum Dis 3D wrote:Why is it that double bonds are shorter than single bonds? Is it due to energy?
Hi, so double bonds are stronger than single bonds, therefore double bonds also have shorter bonds
-
Alessandra Marotta 3L
- Posts: 50
- Joined: Fri Sep 24, 2021 5:17 am
Re: Bond length in double vs single
Double bonds are stronger and therefore shorter than single bonds because it takes more electrons and energy to pull the atoms closer together. Hope this helps!
Re: Bond length in double vs single
Hi, as everyone is saying, a double bond is shorter than a single bond because more electrons are being shared so the force holding the two atoms together is stronger and requires more energy to break it apart!
Re: Bond length in double vs single
Piggybacking from everyone's responses, double bonds are using more electrons (4) as opposed to a single bond which only shares 2 electrons between at atom.
-
Alice Weber 3I
- Posts: 94
- Joined: Fri Sep 24, 2021 7:27 am
Re: Bond length in double vs single
Double bonds are shorter because the double bonds create a stronger attraction between the two atoms compared to a single bonds.
Re: Bond length in double vs single
it creates a stronger atraction between the two atoms thus making it shorter
-
Aryan Gajjar 3D
- Posts: 114
- Joined: Fri Sep 24, 2021 6:05 am
- Been upvoted: 1 time
Re: Bond length in double vs single
Because double bonds are stronger than single bonds, they draw the electrons in the two elements closer together, reducing the bond length.
-
Aaron Kim 1J
- Posts: 106
- Joined: Fri Sep 24, 2021 5:27 am
Re: Bond length in double vs single [ENDORSED]
Double bonds between atoms = more electrons shared between the atoms, so there will be a stronger attraction between the two atoms, pulling them closer toward each other. A single bond between atoms shares less electrons (than a double bond) and thus, the attraction between the atoms is weaker.
-
Reagan Feldman 1D
- Posts: 102
- Joined: Fri Sep 24, 2021 5:44 am
- Been upvoted: 2 times
Re: Bond length in double vs single
As we know, single bonds are characteristically long and weak, while double bonds are shorter and stronger. Double bonds are shorter because there are more bonded electrons, therefore there is a stronger attraction and the atoms are pulled closer together.
-
Michelle Gong
- Posts: 104
- Joined: Fri Sep 24, 2021 7:10 am
Re: Bond length in double vs single
Double bonds are stronger because the atoms are sharing more electrons, so the electrons are also being pulled tighter to the nucleus, thus making them shorter than single bonds. The same is true for triple bonds v. double bonds and single bonds.
-
Jaipal Virdi 2I
- Posts: 105
- Joined: Fri Sep 24, 2021 7:10 am
- Been upvoted: 1 time
Re: Bond length in double vs single
Similar to what others have said, since there are more electrons in a double bond, there is a stronger attraction. That results in the shortened length when compared to a single bond and why they are considered to be stronger.
-
Ashwin Vasudevan 3A
- Posts: 121
- Joined: Fri Sep 24, 2021 5:57 am
Re: Bond length in double vs single
Double bonds are stronger and pull the electrons closer to the atom.
-
Caitlyn Lo 2F
- Posts: 99
- Joined: Fri Sep 24, 2021 6:06 am
Re: Bond length in double vs single
In a double bond, the attraction is stronger, causing the electrons to be pulled closer together and causing the double bond length to be shorter than a single bond length.
-
Lindsey Walter 3E
- Posts: 103
- Joined: Fri Sep 24, 2021 6:13 am
Re: Bond length in double vs single
Double bonds are stronger than single bonds, meaning that there will be a stronger pull between the shared electrons in the double bond. This results in a shorter bond length. Overall, the stronger the bond will be shorter than the weaker bond.
Re: Bond length in double vs single
Double bonds are stronger than single bonds and therefore they have more pull on the electrons that are being shared, as a result of this increased pull on the shared electrons, the bond itself is shorter.
-
Amanda Dankberg 1B
- Posts: 95
- Joined: Fri Sep 24, 2021 5:35 am
Re: Bond length in double vs single
Double bonds are shorter because they share more electrons so they are more attracted to each other and are pulled together
Re: Bond length in double vs single
Double bonds are shorter than single bonds because double bonds are stronger and therefore pull the electrons closer together in the two elements
Re: Bond length in double vs single
Double bonds are shorter than single bonds because they share more electrons which leads to a stronger attraction and thus a stronger pull. This leads to a double bond being shorter compared to a single bond.
-
Anthony_Rio_3K
- Posts: 99
- Joined: Sat Sep 25, 2021 5:04 am
-
Vanessa Wiratmo 3k
- Posts: 103
- Joined: Fri Sep 24, 2021 7:32 am
- Been upvoted: 1 time
Re: Bond length in double vs single
Double bonds have more electrons being shared, meaning that the bond energy is stronger than that of a single bond. With a stronger bond and more electrons being shared, the bond is shorter and therefore becomes harder to break. The electrons are pulled harder and closer.
-
madeleinewright
- Posts: 54
- Joined: Fri Sep 24, 2021 5:36 am
Re: Bond length in double vs single
Since more electrons are shared in double bonds, double bonds have a stronger attraction and therefore the atoms are pulled closer together. Therefore, double bonds are shorter than single bonds.
-
Valerie M Dis 2E
- Posts: 84
- Joined: Fri Sep 24, 2021 6:40 am
Re: Bond length in double vs single
Double bonds are shorter than single bonds because more electrons are being shared, making the bonds stronger. Stronger bonds means that there is a stronger attraction between the molecules, making the bond shorter than a single bond.
-
Madelyn_Rios_2c
- Posts: 110
- Joined: Fri Sep 24, 2021 5:54 am
Re: Bond length in double vs single
Since double bonds share more electrons than a single bond, this makes the bond length shorter. Meaning it is pulling the electrons closer and making it stronger than a single bond.
-
Akshat Katoch 2K
- Posts: 95
- Joined: Fri Sep 24, 2021 5:41 am
Re: Bond length in double vs single
A double bond is going to be stronger than a single bond. A double bond pulls the electrons closer together between the two atoms, compared to a single bond. A double bond shares more electrons so there is a stronger attraction causing them to pull closer together. A single bond is not as strong as a double bond. Bond length is directly related to Bond strength. The shorter the bond the stronger it is.
-
Chloe Fuson
- Posts: 103
- Joined: Fri Sep 24, 2021 5:17 am
Re: Bond length in double vs single
double bonds are shorter because they hold two atoms together more tightly, or closer together, so therefore they are shorter than single bonds.
-
Rachel Bartley 2B
- Posts: 104
- Joined: Fri Sep 24, 2021 6:06 am
Re: Bond length in double vs single
Double bonds are shorter than single bonds because they have more shared electrons between two atoms, meaning there is a greater attraction pulling the two atoms closer together.
-
trevina_brown_2A
- Posts: 105
- Joined: Wed Feb 03, 2021 12:15 am
Re: Bond length in double vs single
Double bonds are stronger and pull the electrons closer to the atom.
-
Harrington Bubb3A
- Posts: 50
- Joined: Fri Sep 24, 2021 6:29 am
Re: Bond length in double vs single
the more electons creates more negativity which causes two atoms to get drawn closer together
-
Edriana J Altea 2G
- Posts: 130
- Joined: Fri Sep 24, 2021 6:18 am
Re: Bond length in double vs single
The bond length in a double bond is shorter than the single because, yes, it does have more energy which draws it closer together. The same trend goes for a triple bond as there is even more energy in that than in a double bond.
-
Esther Kim
- Posts: 99
- Joined: Fri Sep 24, 2021 6:07 am
Re: Bond length in double vs single
Double bonds are stronger and therefore bring the atoms closer together.. so it's shorter
Re: Bond length in double vs single
double bonds usually imply more electrons are being shared, so that means the atoms are being pulled closer, therefore making a double bond shorter than a single bond.
-
Alice Weber 3I
- Posts: 94
- Joined: Fri Sep 24, 2021 7:27 am
Re: Bond length in double vs single
Double bonds are shorter because the atoms have a stronger attraction and are pulled closer together. Single bonds do not have as strong of an attraction.
-
AndreyCastellanos 3H
- Posts: 59
- Joined: Fri Sep 24, 2021 5:46 am
Re: Bond length in double vs single
Double bonds are stronger and therfore shorter, while single bonds are weaker and therefore longer.
Return to “Bond Lengths & Energies”
Who is online
Users browsing this forum: No registered users and 5 guests

