Indicate whether there must be, may be, or cannot be one or more lone pairs of electrons on the central atom:
0--0--0 linear structure with 180 degree bond angles----> may have one or more lone pairs
can anyone explain to me why it is possible that it could have lone pairs? I had assumed that would make the structure no longer linear and would result in a structure more like part a with 120 degree bond angles.
4.1 Lone pairs
Moderators: Chem_Mod, Chem_Admin
-
Ashley Chipoletti 1I
- Posts: 20
- Joined: Fri Sep 29, 2017 7:04 am
Re: 4.1 Lone pairs
If the central atom had 3 lone pairs, and two bonds, the molecule will appear linear.
-
Gianna Apoderado 1B
- Posts: 57
- Joined: Fri Sep 29, 2017 7:04 am
Re: 4.1 Lone pairs
Here is a diagram clarifying what Ashley said above! You can see that the 3 lone pairs and their electron "clouds" have repulsion, such that the bonds between the central atom and the two other atoms are manipulated to a linear shape.
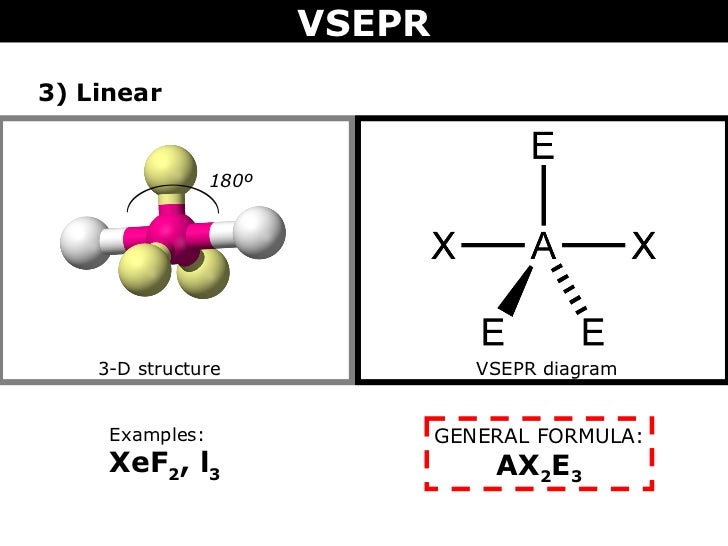

-
Morgan Baxter 1E
- Posts: 50
- Joined: Thu Jul 27, 2017 3:00 am
Re: 4.1 Lone pairs
Yes, Gianna's diagram is really helpful. There are two instances where the shape is linear:
1. 2 areas of electron density where none of which are lone pairs
or
2. 5 regions of electron density where three of which are lone pairs
1. 2 areas of electron density where none of which are lone pairs
or
2. 5 regions of electron density where three of which are lone pairs
-
Swetha Sundaram 1E
- Posts: 59
- Joined: Fri Sep 29, 2017 7:04 am
- Been upvoted: 1 time
Re: 4.1 Lone pairs
But in part b, there are two areas of electron density and it's linear so I thought that there wouldn't be any lone pairs. But the answer says that there may be lone pairs.
-
Alissa Stanley 3G
- Posts: 22
- Joined: Fri Sep 29, 2017 7:06 am
- Been upvoted: 1 time
Re: 4.1 Lone pairs
In the second diagram posted above, can someone please explain the significance of the thicker line and the dotted line?
-
Leanne Wong 1H
- Posts: 50
- Joined: Tue Oct 10, 2017 7:13 am
- Been upvoted: 1 time
Re: 4.1 Lone pairs
Alissa Stanley 3G wrote:In the second diagram posted above, can someone please explain the significance of the thicker line and the dotted line?
The thicker line indicates that the bond is towards us and the dotted line shows that the bond is going away from us. This is to help represent the 3D shape of the molecule which is more informative than just a 2D shape of the molecule.
Return to “Determining Molecular Shape (VSEPR)”
Who is online
Users browsing this forum: No registered users and 6 guests

