On Friday's lecture, the Professor included on the slides, "Polar molecules interact with an electrostatic potential created by cation or anion" I'm not sure what that mean.
I need help on how to determine that a molecule is polar or nonpolar like CCl4 and CHCl3. Thank you!
Polar Bonds versus non polar bonds
Moderators: Chem_Mod, Chem_Admin
-
Alejandro Salazar 1D
- Posts: 31
- Joined: Fri Sep 29, 2017 7:05 am
-
Neha Divi 1K
- Posts: 31
- Joined: Fri Apr 06, 2018 11:02 am
Re: Polar Bonds versus non polar bonds
For determining whether a molecule is polar or not, you need to look at the dipole moments. If the dipole moments are going in opposite directions and cancel each other out then it is non polar. If the dipoles do not cancel it is a polar molecule. Dipole moments occur when there is a separation in charge like those that arise from differences in electronegativity.
-
Bree Perkins 1E
- Posts: 34
- Joined: Fri Apr 06, 2018 11:04 am
Re: Polar Bonds versus non polar bonds
To determine whether a molecule is polar or non-polar, you look at the dipole moments. If there are no dipole moments or the dipole moments cancel each other out, then the molecule is non-polar. If there are dipole moments that don't cancel out, then the molecule is polar. 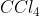 is non-polar because the dipoles cancel each other out.
is non-polar because the dipoles cancel each other out. 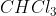 is polar because the dipoles do not cancel out.
is polar because the dipoles do not cancel out.
Hope this helps!
Hope this helps!
Return to “Determining Molecular Shape (VSEPR)”
Who is online
Users browsing this forum: No registered users and 14 guests

