Naming the shape
Moderators: Chem_Mod, Chem_Admin
-
Raquel Rodriguez
- Posts: 68
- Joined: Fri Sep 28, 2018 12:26 am
-
alanaarchbold
- Posts: 68
- Joined: Fri Sep 28, 2018 12:27 am
Re: Naming the shape
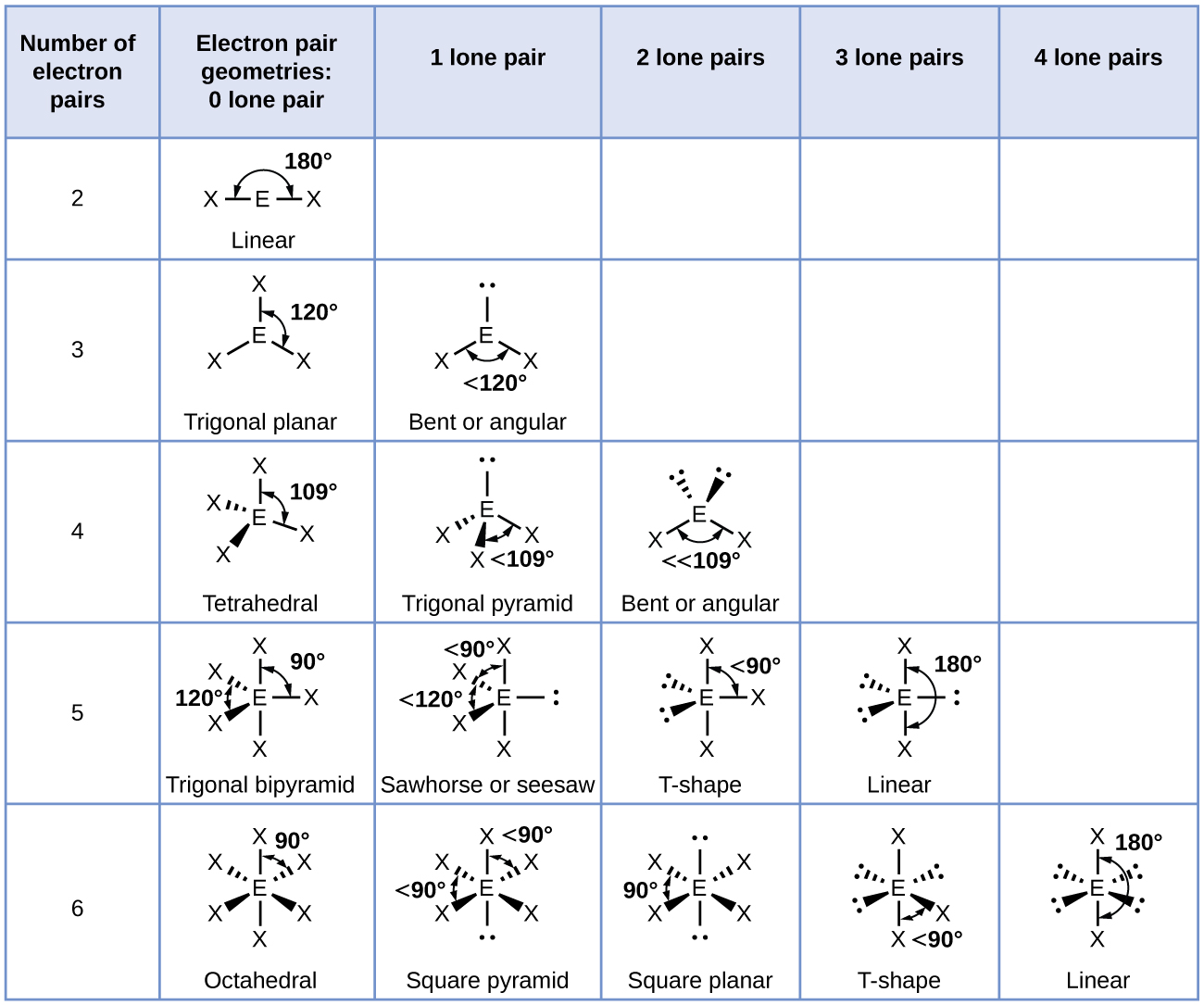
I believe there are 5: linear, trigonal-planar, tetrahedral, trigonal-bipyramidal, and octahedral.
-
Nancy Hu - 4E
- Posts: 30
- Joined: Fri Sep 28, 2018 12:28 am
-
melodyzaki2E
- Posts: 61
- Joined: Fri Sep 28, 2018 12:18 am
Re: Naming the shape
There are images online that are seriously helpful in identifying the different shapes, I use them all the time to make sure I'm doing it right.
-
Maya_Peterson1C
- Posts: 62
- Joined: Fri Sep 28, 2018 12:28 am
Re: Naming the shape
There are also other names of shapes when there are lone pairs such as bent, T-shaped, see-saw, etc.
-
Albert Duong 4C
- Posts: 66
- Joined: Fri Sep 28, 2018 12:17 am
- Been upvoted: 1 time
-
Daniela Alvarado 3B
- Posts: 34
- Joined: Fri Sep 28, 2018 12:26 am
Re: Naming the shape
in class when Lavelle showed us the tetrahedral, why couldn't we put a bond at the bottom? how does putting the last bond pointing toward us affect the atom?
Return to “Determining Molecular Shape (VSEPR)”
Who is online
Users browsing this forum: No registered users and 9 guests