Molecular Shape
Moderators: Chem_Mod, Chem_Admin
-
melodyzaki2E
- Posts: 61
- Joined: Fri Sep 28, 2018 12:18 am
Molecular Shape
When we draw molecules with more than one lone pair around the central atom, how do we depict that that is a trigonal planar or a tetrahedral since its not as obvious as those with atoms attached?
-
Porus_Karwa_2E
- Posts: 72
- Joined: Fri Sep 28, 2018 12:24 am
Re: Molecular Shape
We won't be required to draw a VSEPR model, but a tetrahedral model doesn't have any lone pairs as exhibited in CH4.
-
Albert Duong 4C
- Posts: 66
- Joined: Fri Sep 28, 2018 12:17 am
- Been upvoted: 1 time
Re: Molecular Shape
Usually, when there are lone pairs involved instead of an atom(s), the shape will look similar, but the name and bond angles of the shape are slightly different. For example, instead of 4 atoms, a molecule may have 2 atoms and 2 lone pairs. This is called an angular or bent shape and will have bond angles that are less than 109 degrees because the lone pair's repulsion is greater than a bonding pair. A chart of different shapes: 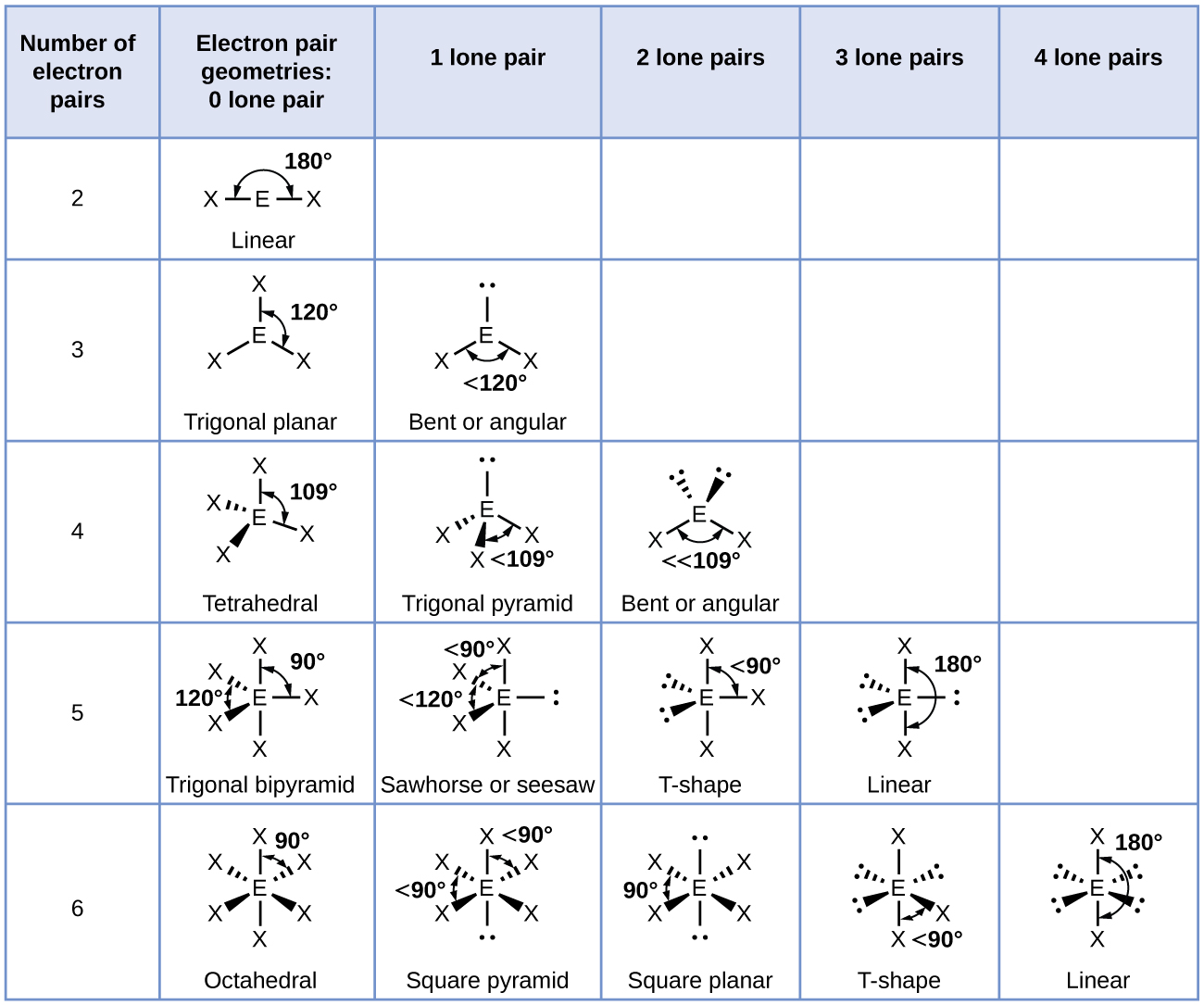

Return to “Determining Molecular Shape (VSEPR)”
Who is online
Users browsing this forum: No registered users and 8 guests

