Page 1 of 1
hybridization
Posted: Mon Nov 18, 2019 11:03 pm
by curry 1E
Hello I am still very confused on hybridization. How do we derive the hybridization by looking at the lewis structure? Or do we have to look at each valence shell of each atom?
Re: hybridization
Posted: Mon Nov 18, 2019 11:18 pm
by Montana James 4G
from what I understand, you look at how many regions of electron density there are and using that number to choose a hybridization scheme. In the end, the number of regions of electron density should equal the number of hybridized orbitals.
Re: hybridization
Posted: Mon Nov 18, 2019 11:42 pm
by BCaballero_4F
You begin with the Lewis Structure which then allows you to use VSEPR to predict the shape, and from these two you should know the number of regions of electron density which would then allow you to derive the hybridization.
Re: hybridization
Posted: Tue Nov 19, 2019 11:34 am
by BNgo_2L
The number of hybrid orbitals are consistent with the number of electron densities of the molecule determined using its VSEPR model. In hybrid orbitals, the max number of orbitals of each shape are s:1, p:3, d:5, etc. The orbitals are then filled using the number of electrons in the atomic orbital of the atom in question.
Re: hybridization
Posted: Tue Nov 19, 2019 12:45 pm
by EvanWang
Look at the lewis structure to help figure out the hybridization of an atom. If a central atom has four areas of electron density around it, then you need a hybridization that corresponds to those four areas. In this case, sp3. If an atom has three areas of electron density, the hybridization is sp2. For Five areas, the hybridization is sp3d, etc.
Re: hybridization
Posted: Thu Nov 21, 2019 1:45 am
by Tauhid Islam- 1H
EvanWang wrote:Look at the lewis structure to help figure out the hybridization of an atom. If a central atom has four areas of electron density around it, then you need a hybridization that corresponds to those four areas. In this case, sp3. If an atom has three areas of electron density, the hybridization is sp2. For Five areas, the hybridization is sp3d, etc.
So the number of electron densities in the central atom of a molecule corresponds to an orbital that is hybridized or a combination of the various orbitals in a particular energy level? Also does that mean that sp^3d can only exist for central atoms that have an energy level of three of higher as an atom in the second energy level can not reach the third energy level? So atoms in period 3 or higher can have hybridized orbitals containing a d or is this not the only case?
Re: hybridization
Posted: Thu Nov 21, 2019 6:28 pm
by Cassandra_1K
how do you go about selecting a hybridization scheme?
Re: hybridization
Posted: Fri Nov 22, 2019 7:33 pm
by Ruth Glauber 1C
^^ What is a step by step method?
Re: hybridization
Posted: Fri Nov 22, 2019 8:07 pm
by Sean Cheah 1E
Ruth Glauber 3L wrote:^^ What is a step by step method?
I don't think there is any kind of step-by-step method. All you need to determine the hybridization scheme of a certain central atom is the number of regions of electron density around that atom (which one can very easily determine using the Lewis dot structure). 2 regions = sp, 3 = sp2, 4 = sp3, 5 = sp3d, etc.
Re: hybridization
Posted: Sat Nov 23, 2019 4:08 pm
by ValerieChavarin 4F
You begin with the Lewis structure that will provide you with the regions of electron density of the center atom. Form their you can determine the hybridization. 2 regions corresponds with
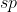
; 3=
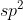
and so on.
Re: hybridization
Posted: Sat Nov 23, 2019 5:51 pm
by RobertXu_2J
Hybridization has to do with molecule shape. You can look at the shape of the molecule to derive the hybridization of the orbitals.
Re: hybridization
Posted: Sat Nov 23, 2019 6:13 pm
by Rodrigo2J
To determine hybridization simply count the number of regions of electron density around the central atom, or the atom in question.