Problem 9C.5 asks: Which of the following ligands can be polydentate? If the ligand can be polydentate, give the maximum number of places on
the ligand that can bind simultaneously to a single metal center: (a) HN(CH2CH2NH2)2
I am unsure how to approach this problem. What is a polydentate?
Polydentate
Moderators: Chem_Mod, Chem_Admin
-
Sebastian Lee 1L
- Posts: 157
- Joined: Fri Aug 09, 2019 12:15 am
- Been upvoted: 1 time
Re: Polydentate
In coordination compounds, a polydentate ligand is a single molecule that can bind to the same central transition metal at multiple sites. This means that it likely will have multiple atoms with lone pairs that can create coordinate covalent bonds with the metal. For this type of question, it's helpful to draw out the structure of the ligand and see how many atoms can create covalent bonds with a lone pair. In the example given in 9C.5 part a, diethylenetriamine is a molecule with 3 nitrogen atoms, each with a lone pair. It can form a ring so that each of those nitrogen atoms is able to bind to a transition metal ion (the compound formed is called a chelate). You can therefore know that HN(CH2CH2NH2)2 can bind at 3 sites, making it tridentate. Again, this is because there are 3 nitrogen atoms that use their lone pair to create a covalent bond with a metal. The carbons and hydrogens form the ring but have no lone pairs to bond with.
-
Jack Riley 4f
- Posts: 100
- Joined: Sat Aug 24, 2019 12:17 am
Re: Polydentate
There are 3 nitrogen atoms in that molecule so it can bind on the central ion at 3 binding sites. Thus, it is tridentate
-
Rebekah Alfred 1J
- Posts: 102
- Joined: Thu Jul 11, 2019 12:15 am
- Been upvoted: 1 time
Re: Polydentate
Here is an example of a polydentate ligand:
Ethylenediaminetetraaceticacid acid (EDTA)
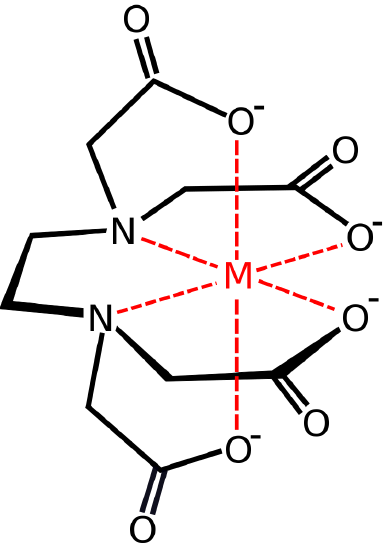
Ethylenediaminetetraaceticacid acid (EDTA)

-
Izzie Capra 2E
- Posts: 103
- Joined: Sat Sep 07, 2019 12:19 am
Re: Polydentate
Yes, and make sure that, when determining if a ligand is polydentate, that a molecule can bind to the SAME central metal atom. If they cannot bind to the same central transition metal, then it is not polydentate and it cannot form chelating complexes.
Who is online
Users browsing this forum: No registered users and 5 guests

