Ignoring second deprotonations
Moderators: Chem_Mod, Chem_Admin
-
SamanthaTolentino 3D
- Posts: 142
- Joined: Wed Sep 30, 2020 10:03 pm
Ignoring second deprotonations
For the textbook question 6E.3, it says that we can ignore the second deprotonations only when the approximation is justified for diprotic acids. When are the approximations justified??
-
Savana Maxfield 3F
- Posts: 199
- Joined: Wed Sep 30, 2020 10:00 pm
Re: Ignoring second deprotonations
I was also wondering this! It seems as if some problems ignore the first deprotonation, while some ignore the second deprotonation (unless I am misinterpreting them). So if anyone knows the answer, it would be greatly appreciated :)
-
Anh Trinh 1J
- Posts: 156
- Joined: Wed Sep 30, 2020 9:55 pm
- Been upvoted: 1 time
Re: Ignoring second deprotonations
The textbook stated to estimate the pH of a polyprotic acid for which all deprotonations are weak by using only the first deprotonation equilibrium and assuming that further deprotonation is insignificant. An exception is sulfuric acid, the only common polyprotic acid that is a strong acid in its first deprotonation.
Another way is to see that if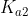 is less than about
is less than about 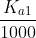 , then the second deprotonation do not affect the pH significantly and can be ignored.
, then the second deprotonation do not affect the pH significantly and can be ignored.
Another way is to see that if
-
Simi Kapila_3E
- Posts: 100
- Joined: Wed Sep 30, 2020 9:40 pm
- Been upvoted: 2 times
Re: Ignoring second deprotonations
I believe you ignore the first deprotonation only when you are dealing with a strong acid. Otherwise, for weak acids, you can ignore the second deprotonation.
-
Eliot Kagan 2G
- Posts: 100
- Joined: Wed Sep 30, 2020 10:08 pm
Re: Ignoring second deprotonations
I think you can ignore the 2nd deprotonation when the 2nd deprotonation is less than a factor of 10^3 from the first deprotonation. It says this in 6E.1 if you want to learn more.
for example:
tartaric acid (weak acid), C2H4O2(COOH)2
ka1= 6.0×10^−4
ka2 = 1.5×10^−5
in this case, you will do the 2nd deprotonation
for example:
tartaric acid (weak acid), C2H4O2(COOH)2
ka1= 6.0×10^−4
ka2 = 1.5×10^−5
in this case, you will do the 2nd deprotonation
-
Naomi Hernandez-Ramirez 1J
- Posts: 70
- Joined: Wed Feb 19, 2020 12:17 am
Re: Ignoring second deprotonations
The first deprotonation is ignored when dealing with a strong acid, for weak acids, ignore the second deprotonation.
Return to “Equilibrium Constants & Calculating Concentrations”
Who is online
Users browsing this forum: No registered users and 10 guests

