A researcher fills a 1.00L reaction vessel with 2.80x10^-5 mol of BrCl gas and heats it to 500K. At equilibrium 8.12% of the BrCl gas remains. Calculate the equilibrium constant Kc assuming the following equation
BrCl <--> Br2 + Cl2
Help!
Also, Is this question related to the material covered for quiz 3?
calculating Kc [ENDORSED]
Moderators: Chem_Mod, Chem_Admin
-
Julianne_Zingmond_1E
- Posts: 22
- Joined: Wed Sep 21, 2016 2:59 pm
- Been upvoted: 1 time
Re: calculating Kc
I won't solve the problem for you, but the idea of this problem is that it gives the initial and final concentrations of BrCl, and therefore you can find the concentrations of the other products at equilibrium and find Kc.The material that will be covered on quiz 3 is only the basics of equilibrium. I think Professor Lavelle said that the first 4 pages in the course reader would be covered.
-
Christopher Reed 1H
- Posts: 33
- Joined: Wed Sep 21, 2016 2:57 pm
- Been upvoted: 1 time
Re: calculating Kc [ENDORSED]
Hi!
I am sure you know how to solve this problem because it's just an ICE box problem is disguise.
I am going to put a disclaimer at the beginning that I cannot figure what sig fig rules the solution uses so my answer will be slightly off. If someone could clarify what rounding needs to occur to get the exact answer, that would be great.
The first step is to balance the chemical equation.
 --> Cl_{2}(g) + Br_{2}(g))
The next step is to determine the initial concentrations of all the reactants and products. The initial concentration of BrCl can be found by dividing 2.80x10^-5 mol by 1.00L. We assume that none of the products have formed yet so their initial concentration would be 0M.
Now we move onto the second row of the ICE box: the change in concentration. This is determined by stoichiometric coefficients which we found in step one. Since we are assuming no products are formed yet we know that the concentration of the product will increase and the concentrations of the reactants will increase. Since we don't really know how much change occurred, we let x equal the amount of change. We use the stoichimetric coefficient to determine the scalar of the change. If you don't like words, below is how the second row of your ICE box should look assuming the columns are in the order denoted by the equation in step 1 (:
Change -2x, +x, +x
Now we must determine the equilibrium concentration. Since we know 8.12% of the BrCl has not been turned into products, that must be how much is left at equilibrium. So we go ahead and take 8.12% of 2.80x10^-5M to get 2.27x10^-6 M. Since we know the initial concentrations of the products was 0 and they increased by x, their final concentration will be x. Below is how your third row of the ICE box will look.
Equilibrium 2.27x10^-6, x, x
Before we move on here is the complete ICE box.
Initial 2.80x10^-5, 0, 0
Change -2x, +x, +x
Equlibrium 2.27x10^-6, x, x
We must now solve for x. We use the first column to calculate x because we have the most information.
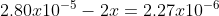
Solving for x we get 1.29x10^-5.
Now we can solve for Kc.
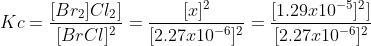
We find the Kc is 32.3. I know that the workbook says 32.1, but I cannot figure out where I did not follow sf rules correctly. If you carry 5sf through the whole problem you get 32.0 If someone could point out the error I would greatly appreciate it. The method however, is correct!
I am sure you know how to solve this problem because it's just an ICE box problem is disguise.
I am going to put a disclaimer at the beginning that I cannot figure what sig fig rules the solution uses so my answer will be slightly off. If someone could clarify what rounding needs to occur to get the exact answer, that would be great.
The first step is to balance the chemical equation.
The next step is to determine the initial concentrations of all the reactants and products. The initial concentration of BrCl can be found by dividing 2.80x10^-5 mol by 1.00L. We assume that none of the products have formed yet so their initial concentration would be 0M.
Now we move onto the second row of the ICE box: the change in concentration. This is determined by stoichiometric coefficients which we found in step one. Since we are assuming no products are formed yet we know that the concentration of the product will increase and the concentrations of the reactants will increase. Since we don't really know how much change occurred, we let x equal the amount of change. We use the stoichimetric coefficient to determine the scalar of the change. If you don't like words, below is how the second row of your ICE box should look assuming the columns are in the order denoted by the equation in step 1 (:
Change -2x, +x, +x
Now we must determine the equilibrium concentration. Since we know 8.12% of the BrCl has not been turned into products, that must be how much is left at equilibrium. So we go ahead and take 8.12% of 2.80x10^-5M to get 2.27x10^-6 M. Since we know the initial concentrations of the products was 0 and they increased by x, their final concentration will be x. Below is how your third row of the ICE box will look.
Equilibrium 2.27x10^-6, x, x
Before we move on here is the complete ICE box.
Initial 2.80x10^-5, 0, 0
Change -2x, +x, +x
Equlibrium 2.27x10^-6, x, x
We must now solve for x. We use the first column to calculate x because we have the most information.
Solving for x we get 1.29x10^-5.
Now we can solve for Kc.
We find the Kc is 32.3. I know that the workbook says 32.1, but I cannot figure out where I did not follow sf rules correctly. If you carry 5sf through the whole problem you get 32.0 If someone could point out the error I would greatly appreciate it. The method however, is correct!
Return to “Equilibrium Constants & Calculating Concentrations”
Who is online
Users browsing this forum: No registered users and 7 guests

