11.37 6th Edition
Moderators: Chem_Mod, Chem_Admin
-
Alexa Tabakian 1A
- Posts: 38
- Joined: Fri Sep 28, 2018 12:20 am
11.37 6th Edition
I was wondering if anyone could explain how to find the partial pressures of N2, H2, and NH3 when only given that K=41. Do we have to involve constants from a table?
-
Cody Do 2F
- Posts: 62
- Joined: Fri Sep 28, 2018 12:23 am
Re: 11.37 6th Edition
We know that for the equation N2 + 3H2 <-> 2NH3 K= 41. When we write it out:
K= PNH32/(PN2)(PH2)3 = 41
For part a, the equation given is 2NH3 <-> N2 + 3 H2. Thus they are looking for K where:
K= (PN2)(PH2)3/PNH32 = 41
When you look at it, this is essentially the inverse of the K value given (41). Thus, to solve part a, you need to do 1/41, which will give a value of 0.024.
For part b, the equation provided is the original equation square rooted, so solve for the square root of 41 = 6.4.
For part c, the equation provided is the original equation squared, so solve for the square of 41, which gives 1.7 x 103
K= PNH32/(PN2)(PH2)3 = 41
For part a, the equation given is 2NH3 <-> N2 + 3 H2. Thus they are looking for K where:
K= (PN2)(PH2)3/PNH32 = 41
When you look at it, this is essentially the inverse of the K value given (41). Thus, to solve part a, you need to do 1/41, which will give a value of 0.024.
For part b, the equation provided is the original equation square rooted, so solve for the square root of 41 = 6.4.
For part c, the equation provided is the original equation squared, so solve for the square of 41, which gives 1.7 x 103
-
Kayla Vo 1B
- Posts: 60
- Joined: Fri Sep 28, 2018 12:26 am
Re: 11.37 6th Edition
In other words for part A, you are looking for the equilibrium constant of the reverse reaction. Since we know that K=41 for the forward reaction, K for the reverse reaction would be the inverse of that of the forward reaction. Thus, the equilibrium constant of the reverse reaction would be 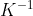 aka
aka
1/41.
Since the coefficients of the reactants and products are halved, the equilibrium constant would equal to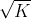 . Therefore, the equilibrium constant would equal to
. Therefore, the equilibrium constant would equal to 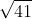 .
.
For part c, the coefficients of the reactants and products are doubled. Therefore, the equilibrium constant equals to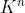 where n equals to 2 since that is the factor. As a result, the equilibrium constant would equal to
where n equals to 2 since that is the factor. As a result, the equilibrium constant would equal to 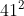 .
.
1/41.
Since the coefficients of the reactants and products are halved, the equilibrium constant would equal to
For part c, the coefficients of the reactants and products are doubled. Therefore, the equilibrium constant equals to
-
Matthew Tran 1H
- Posts: 165
- Joined: Fri Sep 28, 2018 12:16 am
Re: 11.37 6th Edition
Remember that the equilibrium constant is a ratio. That means that you can technically have an infinite number of partial pressures, but their ratio will be the same. Basically, you can have high partial pressures or low partial pressures, but at equilibrium their ratio is equal to the equilibrium constant. So, if you are given only the value of K then you cannot know the partial pressures. If you are given 2 partial pressures then you can find the other partial pressure if there are 3 total products and reactants.
Return to “Equilibrium Constants & Calculating Concentrations”
Who is online
Users browsing this forum: No registered users and 20 guests

