Can someone please explain this problem:
Use the information in Table 11.2 to determine the value of K at 300 K for the reaction 2 BrCl(g) + H2(g) ∆ Br2(g) + 2 HCl(g)
11.39 6th edition
Moderators: Chem_Mod, Chem_Admin
-
Tyra Nguyen 4H
- Posts: 74
- Joined: Fri Sep 28, 2018 12:25 am
Re: 11.39 6th edition
There is a table earlier in the chapter which is needed for reference in this problem. The table will give you the equilibrium constant, K, at various temperatures for specific equilibrium reactions, such as H2(g)+Cl2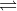 2HCl (g). Knowing the specific equilibrium constants of these referenced equilibrium reactions at certain temperatures, you should be able to find the equilibrium constant of the given reaction in the question at 300K.
2HCl (g). Knowing the specific equilibrium constants of these referenced equilibrium reactions at certain temperatures, you should be able to find the equilibrium constant of the given reaction in the question at 300K.
-
Lynsea_Southwick_2K
- Posts: 55
- Joined: Fri Sep 28, 2018 12:25 am
Re: 11.39 6th edition
Since it is composed of two different equations on the table, how does that work?
-
Samantha Kwock 1D
- Posts: 61
- Joined: Fri Sep 28, 2018 12:24 am
Re: 11.39 6th edition
If a reaction is composed of two different reactions, multiply the K constants to determine the K constant of the third reaction.
-
Nina Do 4L
- Posts: 61
- Joined: Fri Sep 28, 2018 12:27 am
Re: 11.39 6th edition
Answer: Refer to the table referenced earlier in the chapter. It tells you that K equals 377 so you would set 377 equal to your K equation (products over reactions, not including solids or liquids), and then determine K from the table again from the other elements associated with the reaction. see that it is 4.0+10^31. So you would multiply the two K values to get 1.5*10^34.
Return to “Equilibrium Constants & Calculating Concentrations”
Who is online
Users browsing this forum: No registered users and 13 guests

