Taking x away in a Ka calculation
Moderators: Chem_Mod, Chem_Admin
-
Dina Geotas 4A
- Posts: 61
- Joined: Fri Sep 28, 2018 12:29 am
Taking x away in a Ka calculation
Why are we able to disregard the -x when calculating the Ka from an ICE chart?
-
Kristen Kim 2K
- Posts: 70
- Joined: Fri Sep 28, 2018 12:16 am
Re: Taking x away in a Ka calculation
This only applies if the change in composition (x) is less than 5% of the initial value. Usually if Ka is less than 10^-3, I would disregard the x.
-
JiangJC Dis2K
- Posts: 72
- Joined: Fri Oct 12, 2018 12:16 am
Re: Taking x away in a Ka calculation
If you are calculating ka from given concentrations, so the ka is unknown, you employ the 5% rule. You would first calculate the percent ionization by dividing the equilibrium concentration by the initial concentration of one of the products times 100 in order to determine the change in x. If this answer is less than 5%, then the approximation is ok.
-
daniella_knight1I
- Posts: 57
- Joined: Fri Sep 28, 2018 12:18 am
Re: Taking x away in a Ka calculation
If the x in Ka is so small it's insignificant, (less than 5%) then you can disregard it.
-
Miya Lopez 1I
- Posts: 62
- Joined: Tue Nov 14, 2017 3:00 am
- Been upvoted: 1 time
Re: Taking x away in a Ka calculation
In the example Dr. Lavelle did in class today with kA to find the pH, can someone help explain to me how he was able to approximate it to 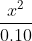 ?
?
-
Emily Ng_4C
- Posts: 65
- Joined: Fri Sep 28, 2018 12:17 am
Re: Taking x away in a Ka calculation
Sometimes, the x is so small that it doesn't have an effect on the overall concentration. For instance, let's say the initial concentration is 1 x 10^-1 and the K value is extremely small, we would know that the numerator/reactants would also have to be extremely small so that the ratio will equal the K value. Thus, the x will not have an effect on the initial concentration (comparatively).
Return to “Equilibrium Constants & Calculating Concentrations”
Who is online
Users browsing this forum: No registered users and 12 guests

