Approximation
Moderators: Chem_Mod, Chem_Admin
-
Cienna Henry 1J
- Posts: 61
- Joined: Fri Sep 28, 2018 12:15 am
- Been upvoted: 1 time
-
Jenna Salas 2H
- Posts: 30
- Joined: Fri Sep 28, 2018 12:22 am
Re: Approximation
You can use approximation when the concentration you calculate is less five percent of the initial concentration.
Re: Approximation
You can only use the approximation when equilibrium constant K is notably small. In class, lavelle told us that in general, when K < 10^-3, we can assume the amount of reactant lost to form product (x) is small enough to ignore.
After using this approximation to solve for x, you have to check it using the 5% rule, which is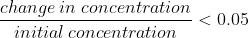
Example:
For the reaction}+3H_{2(g)}\rightleftharpoons 2NH_{3(g)}) with K = 1.64x10^-4 @Temp = 400K
with K = 1.64x10^-4 @Temp = 400K
lets pretend initial concentration of N2= 0.1, H2= 0.2, and NH3 = 0.
We know that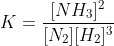
When we set up our Equilibrium equation and ice table, we get^{2}}{(0.1-x)(0.2-3x)^{3}})
That equation looks nasty, but since K is < 10^-3, we can simplify it to^{2}}{(0.1)(0.2)^{3}})
when you solve for x, you want to check that your approximations were valid, so you do:
 and
and 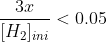
As long as those inequalities are true, you are good to go.
After using this approximation to solve for x, you have to check it using the 5% rule, which is
Example:
For the reaction
lets pretend initial concentration of N2= 0.1, H2= 0.2, and NH3 = 0.
We know that
When we set up our Equilibrium equation and ice table, we get
That equation looks nasty, but since K is < 10^-3, we can simplify it to
when you solve for x, you want to check that your approximations were valid, so you do:
As long as those inequalities are true, you are good to go.
Re: Approximation
Approximate if it follows 5% rule or equilibrium constant is below x10^-3. But I sometimes use quadratic anyway to double check. :)
-
BenJohnson1H
- Posts: 68
- Joined: Fri Sep 28, 2018 12:17 am
-
Hilda Sauceda 3C
- Posts: 76
- Joined: Fri Sep 28, 2018 12:24 am
-
Matthew Casillas 1C
- Posts: 29
- Joined: Fri Sep 28, 2018 12:21 am
-
Cienna Henry 1J
- Posts: 61
- Joined: Fri Sep 28, 2018 12:15 am
- Been upvoted: 1 time
Re: Approximation
Matthew Casillas 1C wrote:Are we allowed to approximate below 10^-4 or 10^-3?
I think I heard 10^-3
Return to “Equilibrium Constants & Calculating Concentrations”
Who is online
Users browsing this forum: No registered users and 28 guests

