% dissociation
Moderators: Chem_Mod, Chem_Admin
-
Jasleen Kahlon
- Posts: 52
- Joined: Wed Nov 21, 2018 12:19 am
Re: % dissociation
Percent dissociation is the amount of H+ ions produced by an acid in solution divided by the amount of unreacted acid [HA].
-
Venus_Hagan 2L
- Posts: 104
- Joined: Fri Aug 02, 2019 12:16 am
Re: % dissociation
percent dissociation is how much of the acid separated into its conjugate base and a proton compared to the original amount of acid or how much of the base separated into its conjugate acid and OH- compared to the original amount of base. You can calculate it by putting either the concentration of the conj acid/base over the original concentration of the acid/base
-
Venus_Hagan 2L
- Posts: 104
- Joined: Fri Aug 02, 2019 12:16 am
Re: % dissociation
Venus_Hagan 2L wrote:percent dissociation is how much of the acid separated into its conjugate base and a proton compared to the original amount of acid or how much of the base separated into its conjugate acid and OH- compared to the original amount of base. You can calculate it by putting either the concentration of the conj acid/base over the original concentration of the acid/base
sorry correction not over the original concentration, its over the concentration of unreacted acid/ base
Re: % dissociation
% disassociation is a measurement of how much an acid has "dissociated" or disconnected into its constituent parts in a solution.
take for example iodic acid
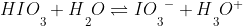
In the solution of H2O , the iodic acid will naturally come apart (based on its Ka) into its constituent base IO3- and H3O+ .
Once there are cations floating around, they act acidically. But importantly, when an acid breaks into parts, that is it losing association: dissociating.
The way that we calculate % dissociation is by dividing the concentration of H3O+ ions over the initial concentration of the acid (in our case HIO3)
take for example iodic acid
In the solution of H2O , the iodic acid will naturally come apart (based on its Ka) into its constituent base IO3- and H3O+ .
Once there are cations floating around, they act acidically. But importantly, when an acid breaks into parts, that is it losing association: dissociating.
The way that we calculate % dissociation is by dividing the concentration of H3O+ ions over the initial concentration of the acid (in our case HIO3)
-
Daria Azizad 1K
- Posts: 116
- Joined: Thu Jul 25, 2019 12:15 am
Re: % dissociation
Calculate the amount of H+ and divide by orginal amount of acid then multiply by 100
Return to “Equilibrium Constants & Calculating Concentrations”
Who is online
Users browsing this forum: No registered users and 9 guests

