If we are given a chemical reaction that has gases involved, do we automatically assume we are calculating KP, or does it depend if we are given values in molar concentrations, or atmospheres/bars?
Thanks!
KC vs KP [ENDORSED]
Moderators: Chem_Mod, Chem_Admin
-
Andrew Wang 1C
- Posts: 101
- Joined: Wed Sep 30, 2020 10:11 pm
- Been upvoted: 5 times
Re: KC vs KP
It would depend if we are given the values of reactants/products in concentration or bars. I remember some problems in the Audio-Visual modules had us calculate Kc when there were only gases in the reaction.
-
SahajDole_1C
- Posts: 106
- Joined: Wed Sep 30, 2020 9:33 pm
Re: KC vs KP
Usually the question should mention what to find but it would depend on the values given.
-
Jonathan Batac - 2D
- Posts: 110
- Joined: Wed Sep 30, 2020 10:03 pm
Re: KC vs KP
I would always check what units are given. As you stated, if given concentration (Molarity) usually you would be calculating for Kc, and if given atm/bars usually you would be calculating for Kp.
-
Gabriel Nitro 1E
- Posts: 101
- Joined: Wed Sep 30, 2020 9:32 pm
- Been upvoted: 1 time
Re: KC vs KP
Hi,
It entirely depends on the context on what you are given. If you are given any information which may lead you to find concentrations (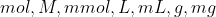 ), use
), use 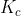 . If you are given any information which may lead you to find partial pressures (
. If you are given any information which may lead you to find partial pressures (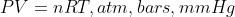 ), then use
), then use 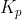 .
.
Hope this helps! :)
It entirely depends on the context on what you are given. If you are given any information which may lead you to find concentrations (
Hope this helps! :)
-
Kareena Patel 1G
- Posts: 102
- Joined: Wed Sep 30, 2020 9:58 pm
- Been upvoted: 1 time
Re: KC vs KP
If the equation only involves gases, I think it is safe to assume that you should use Kp for the problem.
-
Kelly Tran 1J
- Posts: 152
- Joined: Wed Sep 30, 2020 9:50 pm
- Been upvoted: 1 time
Re: KC vs KP [ENDORSED]
The units will tell you whether you are calculating for Kc or Kp. If the molar concentration is given, you are calculating for Kc. If the units of atm/bar is given, you are calculating for Kp.
Return to “Equilibrium Constants & Calculating Concentrations”
Who is online
Users browsing this forum: No registered users and 8 guests

