Converting bar to mol/L
Moderators: Chem_Mod, Chem_Admin
-
Juwan_Madaki_3K
- Posts: 103
- Joined: Wed Sep 30, 2020 9:33 pm
Converting bar to mol/L
I've been having trouble with converting bars to mol/L. Does bar have to be converted to another unit first or can it just be immediately plugged into the ideal gas law equation?
-
Jay Solanki 3A
- Posts: 137
- Joined: Wed Sep 30, 2020 9:59 pm
- Been upvoted: 1 time
Re: Converting bar to mol/L
For the ideal gas equation, the pressure unit is atmospheres. So solve the ideal gas equation for pressure using the given temperature and concentration, and then convert the pressure value from atmospheres to bars. The conversion is about 0.99 atmospheres to 1 bar, so a direct one-to-one conversion from atmospheres to bars is a good conversion. Hope this helps!
-
Neal_Agarwal_3B
- Posts: 54
- Joined: Wed Nov 18, 2020 12:22 am
Re: Converting bar to mol/L
It can be immediately plugged into the ideal gas law equation. If you are trying to find mol/L make sure n/V is on one side of the equation so you solve for molarity. Also be sure to use the appropriate R value as the units of the R value are crucial for the correct answer. I believe you should use the R value of 0.08206 when using bar but correct me if I am wrong. I hope this helped!
-
Isabella Chou 1A
- Posts: 110
- Joined: Wed Sep 30, 2020 9:52 pm
- Been upvoted: 1 time
Re: Converting bar to mol/L
Here's the unit conversion:
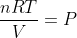
(\frac{L\bullet atm}{K\bullet mol})(K)=atm)
) is the units for the constant R.
is the units for the constant R.
In the lecture today, Dr. Lavelle said 1 bar is equal to approximately 1 atm, so we are usually given pressure in atm even though the SI unit is bar.
In the lecture today, Dr. Lavelle said 1 bar is equal to approximately 1 atm, so we are usually given pressure in atm even though the SI unit is bar.
-
Sabine Salvucci 2E
- Posts: 100
- Joined: Wed Sep 30, 2020 9:42 pm
- Been upvoted: 1 time
Re: Converting bar to mol/L
Hello! The ideal gas law uses atmospheres as the unit for pressure so you would usually want to convert the pressure to atm. Since 1 atm is equal to about 1.01 bar, however, it isn't really necessary to do any conversion calculations; you can just plug the pressure in bar into the ideal gas law. Hope this helps!
-
Karl Yost 1L
- Posts: 179
- Joined: Thu Dec 17, 2020 12:19 am
Re: Converting bar to mol/L
Bar is a pressure unit, while mol/L is a concentration unit. So yes, some conversion will be required. As another commenter already showed (and as Dr. Lavella explained in today's lecture), you can convert partial pressure to concentration by rearranging the ideal gas law. This will leave you with:
n/V=P/RT
For this course, you can use the gas constant for atm, as 1 bar = 1 atm, so you can just plug your values into the equation above to convert bar/atm to a concentration.
n/V=P/RT
For this course, you can use the gas constant for atm, as 1 bar = 1 atm, so you can just plug your values into the equation above to convert bar/atm to a concentration.
Return to “Equilibrium Constants & Calculating Concentrations”
Who is online
Users browsing this forum: No registered users and 7 guests

