Calculating Equilibrium Concentrations
Moderators: Chem_Mod, Chem_Admin
-
Stevie Wisz
- Posts: 59
- Joined: Fri Sep 25, 2015 3:00 am
Calculating Equilibrium Concentrations
When Prof. Lavelle was going over how to calculate the concentrations of products/reactants using ICE Box method I was confused as to how you (for the change in concentration) get -X or +2X, etc. Can someone explain this a little more in depth please? I realize you look at the molar ratio but I just need further clarification. Thank you!
-
William Chwa 1E
- Posts: 20
- Joined: Fri Sep 25, 2015 3:00 am
Re: Calculating Equilibrium Concentrations
X is just a variable that you assign which represents the change in molar concentration for the reactant. The reactant will be used up during the reaction, so the change is represented by -X. Then, as you said, depending on the molar ratios of the products, the product(s)' molar concentration will increase by +nX, where n represents the ratio between the reactant and product.
-
Lisa Leung 2H
- Posts: 23
- Joined: Fri Sep 25, 2015 3:00 am
Re: Calculating Equilibrium Concentrations
An example to illustrate this would be:
For the forward reaction of
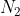 (g)+
(g)+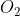 (g)⇌2NO(g)
(g)⇌2NO(g)
In the forward reaction, the change in molar concentration of each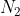 and
and 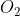 would be -X amount since 1 mole of
would be -X amount since 1 mole of 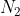 and 1 mole of
and 1 mole of 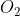 is consumed(negative) for every 2 mole of NO produced. This would mean the change in molar concentration of NO would be +2X since 2 moles of NO is produced(positive) per mole of
is consumed(negative) for every 2 mole of NO produced. This would mean the change in molar concentration of NO would be +2X since 2 moles of NO is produced(positive) per mole of 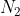 and
and 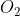 . Like William said above, X is just a variable we assign to the unknown change in molar concentration.
. Like William said above, X is just a variable we assign to the unknown change in molar concentration.
For the forward reaction of
In the forward reaction, the change in molar concentration of each
Return to “Equilibrium Constants & Calculating Concentrations”
Who is online
Users browsing this forum: No registered users and 16 guests

