Hello!
I'm a little confused about how to approach question 5 on our achieve homework this week. I don't really know where to start as it doesn't give us any initial molarities to work with and I'm unsure how to reverse a -log to find the equilibrium molarity. I've pasted the problem below:
The Kb for an amine is 7.054×10−5. What percentage of the amine is protonated if the pH of a solution of the amine is 9.322? Assume that all OH− came from the reaction of B with H2O.
Achieve question #5
Moderators: Chem_Mod, Chem_Admin
-
Alena Zhu 2I
- Posts: 50
- Joined: Wed Feb 17, 2021 12:23 am
Re: Achieve question #5
To start, you would want to find the concentration of OH- ions, which you can find using the pOH of the solution. Since the pH is given, you can find the pOH by subtracting the pH from 14, then using the pOH to find [OH-], which in this case will also be equal to [BH+] (the protonated version of the amine). With the Kb value and these two concentrations using the equation Kb=([OH-][BH+])/[B], you can find [B]. Finally the percent protonated is equal to the concentration of OH- ions divided by the formal concentration, which is the sum of [BH+] and [B], multiplied by 100.
Hope that's helpful!
Hope that's helpful!
-
Melinda Luo 2G
- Posts: 114
- Joined: Fri Sep 24, 2021 6:26 am
Re: Achieve question #5
For this problem, you are actually trying to find the initial concentration of the amine in order to find the percent protonated. While the problem doesn't give a specific base, you would assume the chemical equation looks as follows:
B + H2O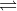 BH+ + OH-
BH+ + OH-
Next, you want to find the [OH-] concentration. Since we are given pH, you simply subtract the pH value from 14, giving you 4.678. To convert from pOH to the concentration of OH-, you simply do 10-pOH, which is approximately 2.1*10-5.
To find the initial concentration of the amine, you would do a "reverse" ICE table, where instead of finding the equilibrium concentration, you're looking for the initial concentration. The table looks as follows:
B BH+ OH-
I x 0 0
C -2.1*10-5 +2.1*10-5 +2.1*10-5
E x-2.1*10-5 2.1*10-5 2.1*10-5
Set up the Kb ratio to find the x value, and then use the values you found for the initial concentration of amine and the concentration of [OH-] to get your answer.
B + H2O
Next, you want to find the [OH-] concentration. Since we are given pH, you simply subtract the pH value from 14, giving you 4.678. To convert from pOH to the concentration of OH-, you simply do 10-pOH, which is approximately 2.1*10-5.
To find the initial concentration of the amine, you would do a "reverse" ICE table, where instead of finding the equilibrium concentration, you're looking for the initial concentration. The table looks as follows:
B BH+ OH-
I x 0 0
C -2.1*10-5 +2.1*10-5 +2.1*10-5
E x-2.1*10-5 2.1*10-5 2.1*10-5
Set up the Kb ratio to find the x value, and then use the values you found for the initial concentration of amine and the concentration of [OH-] to get your answer.
-
Xinyue Zou 2K
- Posts: 102
- Joined: Fri Sep 24, 2021 7:12 am
Re: Achieve question #5
Melinda Luo 2G wrote:For this problem, you are actually trying to find the initial concentration of the amine in order to find the percent protonated. While the problem doesn't give a specific base, you would assume the chemical equation looks as follows:
B + H2OBH+ + OH-
Next, you want to find the [OH-] concentration. Since we are given pH, you simply subtract the pH value from 14, giving you 4.678. To convert from pOH to the concentration of OH-, you simply do 10-pOH, which is approximately 2.1*10-5.
To find the initial concentration of the amine, you would do a "reverse" ICE table, where instead of finding the equilibrium concentration, you're looking for the initial concentration. The table looks as follows:
B BH+ OH-
I x 0 0
C -2.1*10-5 +2.1*10-5 +2.1*10-5
E x-2.1*10-5 2.1*10-5 2.1*10-5
Set up the Kb ratio to find the x value, and then use the values you found for the initial concentration of amine and the concentration of [OH-] to get your answer.
Thank you! I was able to get the right answer :). Could you potentially explain how you set up the reverse ICE table and why 2.1*10^-5 isn't also supplemented for the x value below B?
-
SerenaSabedra
- Posts: 102
- Joined: Fri Sep 24, 2021 7:05 am
Re: Achieve question #5
First, I found the concentration of OH- from the pH. I subtracted the value from 14 for pOH and then calculated 10 to the power of -pOH. This gave me the final concentration and I set up my equation. I set the Kb equal to my final concentration squared divided by x minus the final concentration to represent initial concentration. I multiplied across and made my quadratic equation to solve for x with the quadratic formula. This gives you the initial concentration, which you then use to divide the OH- (final) concentration by the x-value you just found to get the percent protonated.
-
Aditya Desai 1A
- Posts: 145
- Joined: Fri Sep 24, 2021 5:15 am
Re: Achieve question #5
In general, if you know any of the pH, pOH, [H+], or [OH-], you know the other three. useful when things feel overwhelming!
Re: Achieve question #5
I know how to do the math for the problem however I'm still struggling to understand the concept. When doing a problem like this what would be a good indicator that my answer is about right? So more specifically what about this problem indicates that the percentage of amine protonated should be this high?
-
VeronicaShepherd3B
- Posts: 101
- Joined: Wed Feb 17, 2021 12:17 am
Re: Achieve question #5
At low pH, the amino acid is protonated at amine. At high pH, amine groups are deprotonated. At this pH it carries a net positive charge and can be treated as an acid with two pKa's.
-
Melinda Luo 2G
- Posts: 114
- Joined: Fri Sep 24, 2021 6:26 am
Re: Achieve question #5
Xinyue Zou 2K wrote:Melinda Luo 2G wrote:For this problem, you are actually trying to find the initial concentration of the amine in order to find the percent protonated. While the problem doesn't give a specific base, you would assume the chemical equation looks as follows:
B + H2OBH+ + OH-
Next, you want to find the [OH-] concentration. Since we are given pH, you simply subtract the pH value from 14, giving you 4.678. To convert from pOH to the concentration of OH-, you simply do 10-pOH, which is approximately 2.1*10-5.
To find the initial concentration of the amine, you would do a "reverse" ICE table, where instead of finding the equilibrium concentration, you're looking for the initial concentration. The table looks as follows:
B BH+ OH-
I x 0 0
C -2.1*10-5 +2.1*10-5 +2.1*10-5
E x-2.1*10-5 2.1*10-5 2.1*10-5
Set up the Kb ratio to find the x value, and then use the values you found for the initial concentration of amine and the concentration of [OH-] to get your answer.
Thank you! I was able to get the right answer :). Could you potentially explain how you set up the reverse ICE table and why 2.1*10^-5 isn't also supplemented for the x value below B?/quote]
Sure, no problem :).
To set up a "reverse" ICE table, it would essentially be the same as if you were given the initial concentration of the acid/base (like many other problems we've had in the past), but this time, that's the variable we're looking for instead of the equilibrium concentration of [OH-] or [H3O+], like we usually look for.
In the "I" row, B has a concentration of x while BH+ and OH- have a concentration of 0 (since we initially only start with x amount of B).
In the "C" row, B lowers its concentration by y amount (which we will get to later), while BH+ and OH- increase their concentration by the same y amount.
In the "E" row, BH+ and OH- each have an equilibrium concentration of 2.1*10-5, derived from our earlier calculations. Based on what we wrote in the "C" row, BH+ and OH- increased their concentration by y amount while starting from 0, so that means that y must equal 2.1*10-5. Since B lowers its concentration by y amount (based on what we got from the "C" row as well), the equilibrium concentration for B equals x-(2.1*10-5)
Not really sure what you meant by "2.1*10^-5 isn't also supplemented," but the formatting for my table is a bit wonky so it may seem that that number isn't involved for B. However, as I've shown earlier, B changes its concentration by 2.1*10-5, if that's what you're asking.
Hopefully this offers some insight!
-
Ziyi Meng 2K
- Posts: 116
- Joined: Fri Sep 24, 2021 6:48 am
Re: Achieve question #5
Alena Zhu 2I wrote:To start, you would want to find the concentration of OH- ions, which you can find using the pOH of the solution. Since the pH is given, you can find the pOH by subtracting the pH from 14, then using the pOH to find [OH-], which in this case will also be equal to [BH+] (the protonated version of the amine). With the Kb value and these two concentrations using the equation Kb=([OH-][BH+])/[B], you can find [B]. Finally the percent protonated is equal to the concentration of OH- ions divided by the formal concentration, which is the sum of [BH+] and [B], multiplied by 100.
Hope that's helpful!
Hi,
Why is it that the initial/formal concentration has to be [BH+] + [B], but not just the change divided by [B]? I am just getting confused because in the other problems, you can just divide x by the initial moles of acid or base given. Isn't [B] the same thing? Why do we have to add [BH+] to get the correct concentration?
Return to “Equilibrium Constants & Calculating Concentrations”
Who is online
Users browsing this forum: No registered users and 12 guests

