Hi,
I'm not sure what to do with this question? Do you start by writing a chemical reaction equation?
Achieve #8 (due week 3)
Moderators: Chem_Mod, Chem_Admin
-
Simone Byun 1F
- Posts: 101
- Joined: Fri Sep 24, 2021 5:58 am
- Been upvoted: 1 time
Re: Achieve #8 (due week 3)
This problem gives you the Kb, but you're trying to find the pH of an acid. This means that you need to find the Ka, so you would use the concept KaKb = Kw. Once you find that, you can proceed with the problem.
-
Jessica Cornelia Hongarta 1G
- Posts: 114
- Joined: Fri Sep 24, 2021 5:49 am
Re: Achieve #8 (due week 3)
HI! Yes, I find writing the chemical reaction helpful. Then you want to set up the ICE table. Also, you will see that you need Ka instead of Kb to find x. Then, since 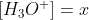 , you can find the pH.
, you can find the pH.
-
Allison Li 2F
- Posts: 103
- Joined: Fri Sep 24, 2021 6:40 am
Re: Achieve #8 (due week 3)
Yes, you would start off by writing the chemical equation and then setting up an ICE table with the initial concentration for NH4+. Because this equation gives off hydronium ions, we need to use Ka instead of Kb to find x, so make sure to convert Kb to Ka by doing 10^-4/Kb. X is hydronium ions, so we can do -log of the value to find the pH.
-
Ginny Ghang 1B
- Posts: 102
- Joined: Fri Sep 24, 2021 7:22 am
Re: Achieve #8 (due week 3)
Use the equation Ka*Kb=Kw to find the value of Ka. You should also set up an ICE table for the reaction: NH4+(aq) <--> H+(aq)+NH3(aq). From the ICE table, you should use Ka=[H+][NH3]/[NH4+]. Solving for x gives you the concentration of H+, which you can then use to find pH.
-
Mara Crooks 1F
- Posts: 53
- Joined: Wed Nov 25, 2020 12:21 am
Re: Achieve #8 (due week 3)
Yeah! You would find the chemical equation and do the basic ICE steps then use the Ka to find the pH.
Return to “Equilibrium Constants & Calculating Concentrations”
Who is online
Users browsing this forum: Google [Bot] and 24 guests

