Hi ya'll! The problem states
"NH3 is a weak base ( Kb=1.8×10−5 ) and so the salt NH4Cl acts as a weak acid. What is the pH of a solution that is 0.013 M in NH4Cl at 25 °C?"
I'm a bit confused on how to create the chemical reaction other than the reactants are NH4CL + H2O. What are tips on how to create the chemical reactions?
Achieve #8 Week 2-3
Moderators: Chem_Mod, Chem_Admin
-
hanniaghernandez
- Posts: 114
- Joined: Fri Sep 24, 2021 6:08 am
Re: Achieve #8 Week 2-3
For this problem, we need to remember that Cl does not affect the pH and neither does h2o. NH4Cl dissociates into the acidic NH4+ ion and the neutral Cl− ion. Therefore the equation for this would be:
NH^+4(aq) ↽−−⇀ H+(aq)+NH3(aq)
hopefully, this helps you solve the problem. After that, you would create your ice table and move on to the steps on how to find the pH.
NH^+4(aq) ↽−−⇀ H+(aq)+NH3(aq)
hopefully, this helps you solve the problem. After that, you would create your ice table and move on to the steps on how to find the pH.
-
Irene Kim 3E
- Posts: 106
- Joined: Fri Sep 24, 2021 6:49 am
Re: Achieve #8 Week 2-3
For this problem, you can think of NH3 and NH4+ as conjugates. The Cl- ion is neutral and does not affect pH. Therefore, by protonating NH3, we can form NH4+ ions, and our equation would be NH3(aq) + H+(aq) 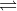 NH4+(aq).
NH4+(aq).
-
Kailin Mimaki 2K
- Posts: 105
- Joined: Fri Sep 24, 2021 5:39 am
Re: Achieve #8 Week 2-3
In buffers, we know that Cl would not affect the pH, so make sure to not include that in your equation. So start with NH4 and add water (H2O). Because NH4 would be an acid, that means it must lose an H+, so NH3 would be on the right side of the equation. And if NH3 was a result of a loss of an H+, then that means an H+ should be added to water to make the correct conjugate acid on the product side of the reaction. Hope this helped!
-
Madison Chan 3B
- Posts: 50
- Joined: Mon Jan 03, 2022 9:17 pm
- Been upvoted: 1 time
Re: Achieve #8 Week 2-3
For this problem, NH3 is the weak base and NH4Cl is the weak acid. However, the salt (NH4Cl) has the anion Cl- which doesn't affect pH so we can omit it from these calculations.
I wrote my equation by putting the salt first and adding water to it on the reactants side. I'm not entirely sure if you're ALWAYS supposed to put the salt first, but that's what has been working for me.The water will then form a bond with one of the hydrogen atoms on NH4, creating NH3 and H3O+ on the products side.
NH4 (aq) + H20 (l) ⇌ NH3 (aq) + H3O+ (aq)
I wrote my equation by putting the salt first and adding water to it on the reactants side. I'm not entirely sure if you're ALWAYS supposed to put the salt first, but that's what has been working for me.The water will then form a bond with one of the hydrogen atoms on NH4, creating NH3 and H3O+ on the products side.
NH4 (aq) + H20 (l) ⇌ NH3 (aq) + H3O+ (aq)
Return to “Equilibrium Constants & Calculating Concentrations”
Who is online
Users browsing this forum: No registered users and 12 guests

