concentration of OH to pH?
Moderators: Chem_Mod, Chem_Admin
-
Nathan Morgan 2C
- Posts: 104
- Joined: Fri Sep 24, 2021 7:16 am
-
Janice Hu 2L
- Posts: 104
- Joined: Fri Sep 24, 2021 5:24 am
- Been upvoted: 2 times
-
Vashe Sundar 3H
- Posts: 109
- Joined: Fri Sep 24, 2021 6:09 am
- Been upvoted: 1 time
Re: concentration of OH to pH?
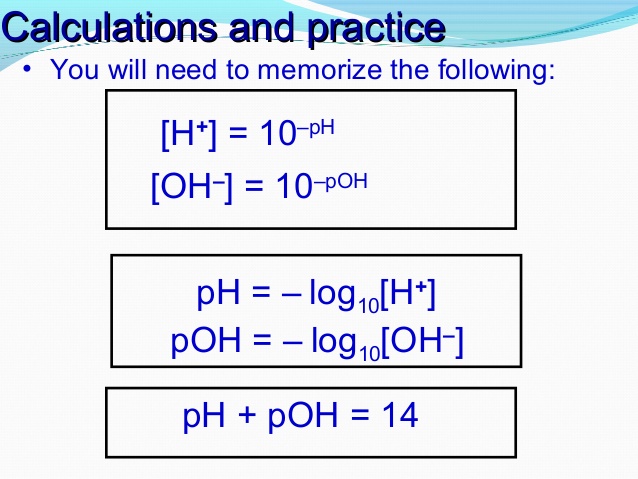
You can use the concentration of OH- to get the pOH, and then we can get the pH by using the relationship between pH and pOH where pH+pOH=14. If you rearrange algebraically, you'll get pH=14-pOH.
-
Jessica Berejikian
- Posts: 100
- Joined: Fri Sep 24, 2021 5:37 am
Re: concentration of OH to pH?
If given the OH concentration, you can convert it to the H concentration using 1x10^-14=[OH][H]. Then, find the pH by taking the -log([H]).
-
KatieWu 1E
- Posts: 102
- Joined: Fri Sep 24, 2021 5:23 am
Re: concentration of OH to pH?
You would first find the concentration of H+ using the equation 10^-14=[OH][H+] and then use the equation pH=-log[H+], or you could find the pOH and then subtract that from 14, since pOH and pH have to add up to 14.
-
Aneesha_Nema_3C
- Posts: 96
- Joined: Fri Sep 24, 2021 5:22 am
Re: concentration of OH to pH?
You have 2 options:
1. convert to pOH and then find pH: pOH = -log [OH-] --> pH = 14 - pOH
2. convert to H+ concentration and then find pH: [H+] = 1x10^-14/[OH] --> pH = -log [H+]
1. convert to pOH and then find pH: pOH = -log [OH-] --> pH = 14 - pOH
2. convert to H+ concentration and then find pH: [H+] = 1x10^-14/[OH] --> pH = -log [H+]
Re: concentration of OH to pH?
pOH and pH will always add to 14 so if you just take the log of concentration of OH and subtract it from 14 you should get pH
-
Parinita Jithendra 2A
- Posts: 101
- Joined: Fri Sep 24, 2021 6:57 am
Re: concentration of OH to pH?
In order to convert the concentration of OH to pH, you can first find pOH by doing -log[OH-]. Using this, you can subtract it by 14 to find pH.
-
Tylina Guo 1K
- Posts: 101
- Joined: Fri Sep 24, 2021 5:30 am
- Been upvoted: 1 time
Re: concentration of OH to pH?
concentration of OH times concentration of H+ is equal to 1x10^-14. Use that to determine [H+], and then pH is the -log([H+]).
Re: concentration of OH to pH?
You do -log(OH) to go get the pOH. Then to get PH you just subtract 14-pOH.
-
Tristan Friet 3G
- Posts: 100
- Joined: Wed Feb 17, 2021 12:23 am
Re: concentration of OH to pH?
Using the concentration of OH, you can plug it into the equation -log[OH-] = pOH, giving you the pOH. Then, knowing the relationship between pH and pOH, you would do 14 - pOH = pH, to get your pH.
-
andrea tarelo 3e
- Posts: 98
- Joined: Fri Sep 24, 2021 6:43 am
-
Saebean Yi 3E
- Posts: 101
- Joined: Fri Sep 24, 2021 6:57 am
Re: concentration of OH to pH?
The easiest way is to calculate pOH, by using -log[OH-]. Then, since pH and pOH always add to 14, just subtract the pOH by 14 and that should be the pH.
-
Isamar Aburto Paniagua 2K
- Posts: 51
- Joined: Wed Feb 20, 2019 12:18 am
-
Kavya Anand 2B
- Posts: 101
- Joined: Fri Sep 24, 2021 5:25 am
Re: concentration of OH to pH?
If given the concentration of OH, u can use the equations pOH = -log OH- and pH + pOH = 14 to convert the concentration from OH to pOH then pH.
-
Ainsley McCabe 2D
- Posts: 101
- Joined: Fri Sep 24, 2021 6:54 am
Re: concentration of OH to pH?
Take the negative log of the OH- concentration, and then do 14 - that number to get the pH
-
Michael 1G
- Posts: 101
- Joined: Fri Sep 24, 2021 6:16 am
- Been upvoted: 1 time
Re: concentration of OH to pH?
If you were given an [OH-] concentration and wanted it in terms of pH you could find the pOH by taking the -log of the concentration. From there, you would need to subtract the pOH from 14 to be left with the pH.
Return to “Equilibrium Constants & Calculating Concentrations”
Who is online
Users browsing this forum: No registered users and 2 guests