strong acids and bases
Moderators: Chem_Mod, Chem_Admin
-
Ruiting Jia 4D
- Posts: 65
- Joined: Fri Sep 28, 2018 12:27 am
strong acids and bases
Can someone give a quick refresher on how to identify strong acids and bases vs weak acids and bases, and are there pages in the textbook to also help with this? I understand how the two differ when it comes to dissociating in the chemical equations.
-
AngelaZ 1J
- Posts: 65
- Joined: Fri Sep 28, 2018 12:23 am
Re: strong acids and bases
The strong acids and bases can be found on F83 in the 6th edition textbook:
Strong acids: HBr, HCl, HI, HNO3, NClO4, HClO3, H2SO4
Strong bases: Group 1 hydroxides, alkaline earth metal hydroxides (Ca(OH)2, Sr(OH)2, Ba(OH)2), Group 1 and Group 2 oxides
Strong acids: HBr, HCl, HI, HNO3, NClO4, HClO3, H2SO4
Strong bases: Group 1 hydroxides, alkaline earth metal hydroxides (Ca(OH)2, Sr(OH)2, Ba(OH)2), Group 1 and Group 2 oxides
-
Jonathan Omens 1K
- Posts: 60
- Joined: Fri Sep 28, 2018 12:15 am
Re: strong acids and bases
If you are given a K value, an acid with a Ka < 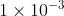 or a base with Kb less than that value will be a weak acid/base respectively.
or a base with Kb less than that value will be a weak acid/base respectively.
(This is also a general rule but works in the majority of situations)
(This is also a general rule but works in the majority of situations)
Return to “Non-Equilibrium Conditions & The Reaction Quotient”
Who is online
Users browsing this forum: No registered users and 6 guests

