For this problem we are only looking at the change in the P or R values of the Q equation, using K as a fixed ratio.
Your K value is the fixed ratio between the left and right sides when at equilibrium. While Q is also a ratio between the [R] and [P] but the ratio might not be at equilibrium.
This means that when K is larger than Q, there are more reactants readily available to become products. When Q is larger than K, there is more product readily available to become reactants.
K=
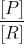
Q=

So, if K>Q then the reaction is moving forward, towards the products. In order to reach equilibrium more product must form.
This makes [R]>[P] because if Q is small, then R must be larger than the P. This would cause the reaction to occur in the direction of the products, to negate the effects of a large concentration of reactants.
K=

Q=

If K<Q the reaction is moving in reverse, towards the reactants. This implies that there is more product available than reactant and to reach equilibrium, the reaction must work in reverse.
This makes [P]>[R] because if Q is larger than K, the numerator P of the Q equation, must be larger than the R. When the amount of product is large, it is necessary for the reaction to occur in reverse so that equilibrium might be met.