When Q=K
Moderators: Chem_Mod, Chem_Admin
-
OishiBhattacharya2K
- Posts: 100
- Joined: Fri Sep 24, 2021 6:03 am
When Q=K
Just for clarification, when Q=K, is it safe to assume that the reaction is at equilibrium?
-
Chloe Fuson
- Posts: 103
- Joined: Fri Sep 24, 2021 5:17 am
Re: When Q=K
Yes, if you start out solving for Q because you are unsure whether a reaction is at equilibrium, and then find that the value you calculate is equal to K, then you now know the reaction is at equilibrium.
-
alexjung1A
- Posts: 101
- Joined: Fri Sep 24, 2021 7:07 am
-
Emily Nguyen 3L
- Posts: 49
- Joined: Mon Jan 03, 2022 9:44 pm
- Been upvoted: 1 time
Re: When Q=K
Yes, if the Q (reaction quotient) value that calculates the ratio of product to reactant concentration is equal to the K (equilibrium constant) value, then the reaction is at equilibrium.
-
Terrence Chi
- Posts: 99
- Joined: Fri Sep 24, 2021 6:06 am
Re: When Q=K
Hi, if Q=K, then the system is at equilibrium since the concentration of product over reactant is equal to the equilibrium constant K. Hope this helps!
-
Sidney Shah 3H
- Posts: 100
- Joined: Fri Sep 24, 2021 5:47 am
- Been upvoted: 1 time
Re: When Q=K
Yes, since Q is the ratio of products to reactants, if that ratio is equal to K, then you can assume rxn is at equilibrium
-
Jane Wang 1E
- Posts: 115
- Joined: Fri Sep 24, 2021 6:36 am
-
Jacqueline Vargas 3L
- Posts: 113
- Joined: Wed Feb 17, 2021 12:22 am
Re: When Q=K
Yes. This question is relevant when we are studying a reaction. We can compare a given K value to the a Q value based on the information we have of the reaction (concentration of products and reactants) in order to determine if the reaction has reached equilibrium or if it has not, which side of the reaction will it favor.
-
Kathryn Heinemeier 3H
- Posts: 104
- Joined: Fri Sep 24, 2021 5:09 am
-
Alekhya_Pantula_2E
- Posts: 100
- Joined: Fri Sep 24, 2021 6:37 am
- Been upvoted: 1 time
Re: When Q=K
Yes essentially Q is used as a measure to see if the reaction has reached equilibrium, so once Q=K then we can confirm that the reaction is at equilibrium.
-
Dillon Taing 3H
- Posts: 102
- Joined: Fri Sep 24, 2021 5:20 am
Re: When Q=K
Q is the product to reactant ratio at any given time in a reaction while K is the product to reactant ratio at equilibrium. When Q=K, then neither side of the system is favored and the reaction is in equilibrium.
-
Sasha Gladkikh 2A
- Posts: 190
- Joined: Fri Sep 24, 2021 5:50 am
- Been upvoted: 3 times
Re: When Q=K
Hi,
When Q = K, the reaction is at equilibrium—and will remain so until the system is disturbed (e.g. if heat is added or the pressure is increased).
Here is a diagram that illustrates the comparison between the reaction quotient (Q) and equilibrium constant (K):
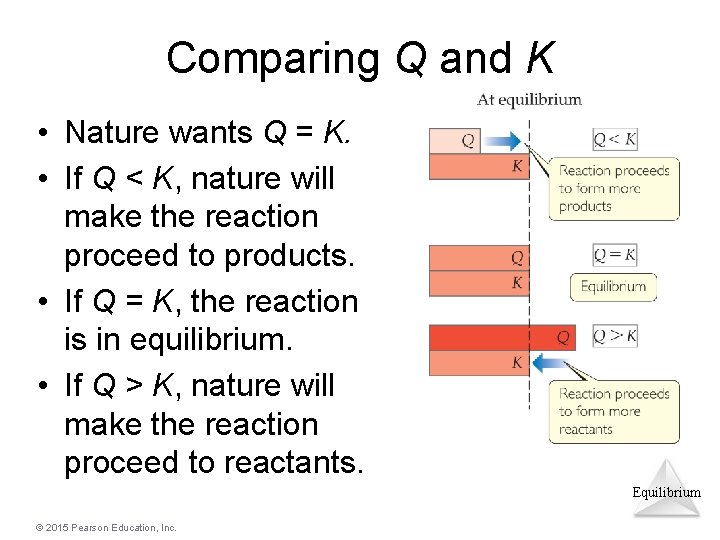
When Q = K, the reaction is at equilibrium—and will remain so until the system is disturbed (e.g. if heat is added or the pressure is increased).
Here is a diagram that illustrates the comparison between the reaction quotient (Q) and equilibrium constant (K):

-
Alena Zhu 2I
- Posts: 50
- Joined: Wed Feb 17, 2021 12:23 am
Re: When Q=K
Yep! The reaction quotient is usually measured and calculated multiple times as a reaction precedes, and when it finally equals K, it indicates that the system is at equilibrium and the reactions are occurring at the same rate.
-
Aaron Martinez
- Posts: 86
- Joined: Fri Sep 24, 2021 6:54 am
Re: When Q=K
Yes, if Q=K, then the reaction is at equilibrium. This will always be the case. If Q does not equal K, then it's not at equilibrium.
-
Madison Kiggins 1E
- Posts: 102
- Joined: Fri Sep 24, 2021 5:41 am
Re: When Q=K
Yes, if Q is equal to K that means that the reaction is at equilibrium. If Q is any other value besides K it is not at equilibrium.
-
Joseph Lee
- Posts: 100
- Joined: Fri Sep 24, 2021 6:35 am
Re: When Q=K
Yep! When the reaction quotient is the same thing as the equilibrium constant, then the reaction is at equilibrium.
-
Mia Orr 3B
- Posts: 103
- Joined: Fri Sep 24, 2021 7:11 am
Re: When Q=K
Yes, when Q is equal to K it means that the reaction is at equilibrium. When Q is any other value, it means that the reaction is not at equilibrium. Hope this helps!
-
Caitlyn Lo 2F
- Posts: 99
- Joined: Fri Sep 24, 2021 6:06 am
-
Samir Panwar
- Posts: 111
- Joined: Fri Sep 24, 2021 6:26 am
- Been upvoted: 1 time
Re: When Q=K
Yes, if the ratio of products to reactants equals K then we can say Q=K, which means the reaction is at equilibrium.
-
Tanvi Akula 2K
- Posts: 95
- Joined: Fri Sep 24, 2021 6:41 am
-
Amy Huynh 1B
- Posts: 103
- Joined: Fri Sep 24, 2021 6:58 am
Re: When Q=K
Yes, this is the case as K is the numerical value of Q at the "end" of the reaction when equilibrium has been reached. Hope that helps!
-
Ramya_Paravastu_1H
- Posts: 54
- Joined: Mon Jan 03, 2022 10:55 am
Re: When Q=K
Yes! K is the ratio of the concentrations of the products and reactants at equilibrium. When the reaction quotient (the same ratio, but at any given point in time or progression of the reaction) is equal to K, the system is at equilibrium.
-
Mya_Chiarappa_2C
- Posts: 99
- Joined: Fri Sep 24, 2021 7:15 am
Re: When Q=K
Yes, Q is basically the same as K in how it is calculated, it is just called K at equilibrium and Q when not at equilibrium. When K = Q it is at equilibrium.
-
Collin Le 3I
- Posts: 52
- Joined: Mon Jan 03, 2022 9:35 pm
Re: When Q=K
Yes, Q and K are calculated the same way, so if Q is equal to K, the system is at equilibrium.
-
Nicole Friday 1E
- Posts: 51
- Joined: Mon Jan 03, 2022 10:45 am
-
Rebekah Jung 1C
- Posts: 101
- Joined: Fri Sep 24, 2021 6:40 am
- Been upvoted: 1 time
Re: When Q=K
Yes, when Q=K the reaction is at equilibrium. If Q<K then we can predict the reaction will favor the products until K is reached. When the calculated Q value > K then we can predict the reactants will be favored, going right to left.
-
William Huang 1K
- Posts: 111
- Joined: Fri Sep 24, 2021 7:35 am
Re: When Q=K
Yes, Q will proceed to move in the direction that gets the system to equilibrium (when Q=K).
-
Amanda Pineda 3H
- Posts: 102
- Joined: Fri Sep 24, 2021 6:43 am
Re: When Q=K
If Q=K then yes this means that the reaction is at equilibrium. The reaction quotient, Q, will equal the equilibrium constant,K, meaning the reaction itself is at a balance.
-
Emilie Otterson 1C
- Posts: 11
- Joined: Wed Nov 25, 2020 12:21 am
Re: When Q=K
Yes, if Q=K then the reaction must be at equilibrium. Since K is the equilibrium constant (signifying the reaction is at equilibrium), if the reaction quotient, Q, is equal to it, then the reaction has reached equilibrium.
-
Achyutha Kodavatikanti_3H
- Posts: 51
- Joined: Mon Jan 03, 2022 9:33 pm
Re: When Q=K
Yes, when Q = K the reaction is considered to be at equilibrium. Q measures the relative product and reactant concentrations at a specific point in time, which may or may not be equal to the product and reactant concentrations at equilibrium (K).
-
Myra Goraya Dis 2E
- Posts: 102
- Joined: Fri Sep 24, 2021 6:24 am
Re: When Q=K
Both Q and K are derived through the concentrations of products/reactants. If Q and K are equivalent that means the system is at equilibrium and the value is no longer "Q" but instead it is "K"
-
Clarence Clavite 2K
- Posts: 100
- Joined: Fri Sep 24, 2021 5:33 am
-
Vanessa Bartoli 1C
- Posts: 52
- Joined: Mon Jan 03, 2022 10:34 am
Re: When Q=K
Yes, when Q is = to K, it indicates that the reaction is at equilibrium. Conversely, we know that when q is not = K, the reaction is not at equilibrium.
-
Neha Mukund
- Posts: 109
- Joined: Fri Sep 24, 2021 5:23 am
-
Caitlin_Doak_2H
- Posts: 52
- Joined: Wed Feb 17, 2021 12:18 am
Re: When Q=K
If the Q is a big number because the numerator is large, that means there are more products so it would need to shift left towards reactants. If it is a smaller number, then Q would be more reactant heavy and shift to the right toward the products.
-
kayleec1004
- Posts: 74
- Joined: Fri Sep 24, 2021 6:57 am
-
Jillian Sarquiz- 2B
- Posts: 113
- Joined: Fri Sep 24, 2021 6:29 am
-
Acharya Ranawat 3E
- Posts: 102
- Joined: Fri Sep 24, 2021 6:39 am
Re: When Q=K
Yes. You can assume that it is at equilibrium since the ratios of the concentrations are the same. Hope this helps!
-
JennyZhu1K
- Posts: 101
- Joined: Fri Sep 24, 2021 7:12 am
-
Esmeralda_Solis_2D
- Posts: 102
- Joined: Fri Sep 24, 2021 5:00 am
-
RCortez_1A
- Posts: 26
- Joined: Wed Feb 17, 2021 12:20 am
Re: When Q=K
Yes, when Q = K it is safe to assume that the reaction is at equilibrium since K is used only when the reaction is at equilibrium.
-
Michael 1G
- Posts: 101
- Joined: Fri Sep 24, 2021 6:16 am
- Been upvoted: 1 time
Re: When Q=K
Yes. Since k is the constant for the reaction at equilibrium, if Q is equal to K it must mean that equilibrium has been reached.
-
Jessica Servoss 1H
- Posts: 92
- Joined: Fri Sep 24, 2021 7:07 am
- Been upvoted: 1 time
Re: When Q=K
When you are checking if a reaction has reached equilibrium and you compare Q to K (which would be given), and Q=K, the reaction is at equilibrium.
-
Jessica Sun 2I
- Posts: 102
- Joined: Fri Sep 24, 2021 7:18 am
Re: When Q=K
Only when the reaction quotient(Q) equals the equilibrium constant(K) will the reaction be in equilibrium.
-
Sabira Mohammed 3I
- Posts: 100
- Joined: Fri Sep 24, 2021 5:34 am
-
Anna Furton
- Posts: 103
- Joined: Fri Sep 24, 2021 6:35 am
Re: When Q=K
Yes, when Q=K, the reaction is at equilibrium. This is because K is the ratio of product concentration over reactant concentration at equilibrium, and Q is the exact same thing but when the reaction is at an undetermined state. Therefore, when they are equal, the reaction state is determined to be equilibrium.
-
Alice Weber 3I
- Posts: 94
- Joined: Fri Sep 24, 2021 7:27 am
Re: When Q=K
Yep. K is the equilibrium constant so if Q is the same as K, you assume the reaction is in equilibrium.
-
indigoaustin 3H
- Posts: 101
- Joined: Fri Sep 24, 2021 5:48 am
Re: When Q=K
Yes, when Q=K the system is at equilibrium. The formulas for Q and K are the same, K just specifies the concentrations/pressures are at equilibrium.
-
Jaipal Virdi 2I
- Posts: 105
- Joined: Fri Sep 24, 2021 7:10 am
- Been upvoted: 1 time
Re: When Q=K
Yup! if q=k, it is at equilibrium since they use the same formula, the only difference is the context in which you label them.
-
Daniela G 2C
- Posts: 92
- Joined: Fri Sep 24, 2021 5:06 am
Re: When Q=K
Although a very rare instance, when Q=K, it is safe to assume that the reaction is at equilibrium.
-
Samantha Toscano 2C
- Posts: 106
- Joined: Wed Feb 17, 2021 12:24 am
-
Baffour Adusei 1L
- Posts: 113
- Joined: Fri Sep 24, 2021 6:51 am
- Been upvoted: 2 times
-
Skylar Lo 2C
- Posts: 110
- Joined: Fri Sep 24, 2021 5:10 am
-
Kayla Ziebell 1H
- Posts: 99
- Joined: Fri Sep 24, 2021 6:39 am
Re: When Q=K
Yes if you have K, and solve the ratio of products over reactants and find that Q is equal to K, then you can assume that the reaction is at equilibrium.
-
Ashrita Singh 2F
- Posts: 111
- Joined: Fri Sep 24, 2021 6:33 am
-
Holly Do 2J
- Posts: 104
- Joined: Fri Sep 24, 2021 5:11 am
Re: When Q=K
Yes the reaction is at equilibrium when Q=K. This is because K is the equilibrium constant while Q is the ratio when the reaction is NOT at equilibrium therefore if Q DOES equal K, the ratio is equal to the equilibrium ratio making it at equilibrium.
-
trevina_brown_2A
- Posts: 105
- Joined: Wed Feb 03, 2021 12:15 am
-
elliemehrara
- Posts: 56
- Joined: Fri Sep 24, 2021 5:16 am
-
Eszter Kovacs 1A
- Posts: 100
- Joined: Fri Sep 24, 2021 5:41 am
-
Hannah Thornton 1F
- Posts: 104
- Joined: Fri Sep 24, 2021 5:53 am
-
Amy Jordan 2A
- Posts: 104
- Joined: Fri Sep 24, 2021 6:23 am
Re: When Q=K
Hi, yes when Q=K the reaction is at equilibrium. You can compare the two to see which side is favored as well. Hope this helps!
-
Madison Rhynhart 3H
- Posts: 99
- Joined: Fri Sep 24, 2021 6:20 am
Re: When Q=K
Yes ! this is true because if Q is the ratio of products to reactants and K is the ratio of those at equilibrium and then Q =K then we can assume the reaction is at equilibrium.
-
Talia Tam 3L
- Posts: 107
- Joined: Fri Sep 24, 2021 5:38 am
-
Nicole Friday 1E
- Posts: 51
- Joined: Mon Jan 03, 2022 10:45 am
-
Madeline Ellmore 2C
- Posts: 25
- Joined: Wed Feb 19, 2020 12:18 am
Return to “Non-Equilibrium Conditions & The Reaction Quotient”
Who is online
Users browsing this forum: No registered users and 10 guests

