Exo/Endo reactions
Moderators: Chem_Mod, Chem_Admin
-
Amy Kalteis 2F
- Posts: 100
- Joined: Fri Sep 24, 2021 7:05 am
Exo/Endo reactions
Hi! Can someone outline what (reactants or products) are favored and when during an exothermic and endothermic reaction? (or where would I be able to find this in the textbook?)
-
Mia Yamada 1J
- Posts: 104
- Joined: Fri Sep 24, 2021 5:08 am
Re: Exo/Endo reactions
Hi Amy!
I'm sorry I'm not exactly sure where you would find this information in the textbook, but here is what Professor Lavelle went over in, I believe, the last lecture of Week 1.
If a reaction is endothermic, meaning it requires heat while forming product, then heating will favor the formation of the products.
If a reaction is exothermic, meaning it gives off heat while forming product, then heating will favor the formation of the reactants.
What tends to help me in understanding this concept is treating heat as a component of the reaction (so either a product or reactant, depending on whether its exo or endo).
For instance, in an endothermic reaction, heat would be placed on the reactants' side, since heat is absorbed and used in the reaction. So, if you were to increase the temperature of the reaction, then you would essentially be increasing the amount of heat/reactants, so the reaction would shift toward the products to accommodate for this change in temperature and reestablish equilibrium. :)
I'm sorry I'm not exactly sure where you would find this information in the textbook, but here is what Professor Lavelle went over in, I believe, the last lecture of Week 1.
If a reaction is endothermic, meaning it requires heat while forming product, then heating will favor the formation of the products.
If a reaction is exothermic, meaning it gives off heat while forming product, then heating will favor the formation of the reactants.
What tends to help me in understanding this concept is treating heat as a component of the reaction (so either a product or reactant, depending on whether its exo or endo).
For instance, in an endothermic reaction, heat would be placed on the reactants' side, since heat is absorbed and used in the reaction. So, if you were to increase the temperature of the reaction, then you would essentially be increasing the amount of heat/reactants, so the reaction would shift toward the products to accommodate for this change in temperature and reestablish equilibrium. :)
Last edited by Mia Yamada 1J on Mon Jan 17, 2022 6:08 pm, edited 1 time in total.
-
Vivek Chotai 2C
- Posts: 138
- Joined: Fri Sep 24, 2021 7:25 am
- Been upvoted: 1 time
Re: Exo/Endo reactions
In an endothermic reaction, where delta H is positive, heating the system would favor the products. Cooling would favor the reactants.
In an exothermic reaction, where delta H is negative, heating the system would favor the reactants. Cooling would favor the products.
You can think of an endothermic reaction as reactants + heat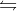 products. So increasing the left side (heating) would shift the equilibrium to the right.
products. So increasing the left side (heating) would shift the equilibrium to the right.
And likewise you can think of an exothermic reaction as reactants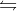 products + heat. So increasing the heat on the right side would shift the equilibrium to the left.
products + heat. So increasing the heat on the right side would shift the equilibrium to the left.
In an exothermic reaction, where delta H is negative, heating the system would favor the reactants. Cooling would favor the products.
You can think of an endothermic reaction as reactants + heat
And likewise you can think of an exothermic reaction as reactants
-
Christine Lin 1H
- Posts: 108
- Joined: Fri Sep 24, 2021 5:24 am
Re: Exo/Endo reactions
Additionally, if the enthalpy is negative, this represents an exothermic reaction, where an increase in temperature causes a decrease in the K value.
If enthalpy is positive for a reaction, it would be endothermic, and an increase in temperature causes an increase of the K value.
If enthalpy is positive for a reaction, it would be endothermic, and an increase in temperature causes an increase of the K value.
-
Quade Albert 2J
- Posts: 102
- Joined: Fri Sep 24, 2021 6:09 am
Re: Exo/Endo reactions
Endothermic favors products.
Exothermic favors reactants.
I remember this because endo reminds me of enter and the system requires new entering energy to form products. Exo reminds me of exit, releasing energy and favoring reactants.
Exothermic favors reactants.
I remember this because endo reminds me of enter and the system requires new entering energy to form products. Exo reminds me of exit, releasing energy and favoring reactants.
-
Aditya Desai 1A
- Posts: 145
- Joined: Fri Sep 24, 2021 5:15 am
Re: Exo/Endo reactions
One way to think of it is as heat being a reactant or product, and then using Le Chatelier's principle.
-
Martha Avila 1I
- Posts: 100
- Joined: Fri Sep 24, 2021 6:21 am
- Been upvoted: 1 time
Re: Exo/Endo reactions
Hello. So when you have a release of energy this is known as an exothermic equation and your delta H is negative. This reaction will favor the reactants. Vise versa when you have an equation that require energy it is an endothermic reaction and your delta H is positive. This reaction will favor the products. Hope this helps.
-
Emily Nguyen 3L
- Posts: 49
- Joined: Mon Jan 03, 2022 9:44 pm
- Been upvoted: 1 time
Re: Exo/Endo reactions
Hi Amy!
During exothermic reactions, heat is released and K decreases to favor reactants. This is to release less heat (limit the forward reaction), which helps offset the rise in temperature (aligning with Le Chatelier's principle). During endothermic reactions, heat is absorbed and K increases to favor products. This is to absorb more heat and help offset the increase in temperature resulting from the reverse exothermic reaction (also Le Chatelier's principle).
You can find more information about this on pg. 431 in the textbook in section 5J.3: Temperature and Equilibrium.
During exothermic reactions, heat is released and K decreases to favor reactants. This is to release less heat (limit the forward reaction), which helps offset the rise in temperature (aligning with Le Chatelier's principle). During endothermic reactions, heat is absorbed and K increases to favor products. This is to absorb more heat and help offset the increase in temperature resulting from the reverse exothermic reaction (also Le Chatelier's principle).
You can find more information about this on pg. 431 in the textbook in section 5J.3: Temperature and Equilibrium.
-
Amanda Pineda 3H
- Posts: 102
- Joined: Fri Sep 24, 2021 6:43 am
Re: Exo/Endo reactions
If a reaction is endothermic, it requires heat and it will favor the formation of products. If a reaction is exothermic, then heating will favor the formation of reactants. An increase in temperature for an endothermic reaction will cause an increase in K. An increase in temperature for an exothermic reaction will cause a decrease in K.
-
Matthew Vu 3C
- Posts: 109
- Joined: Fri Sep 24, 2021 5:07 am
- Been upvoted: 1 time
Re: Exo/Endo reactions
When a reaction is endothermic, energy is being invested. Think of it as a reactant, so if you're increasing temperature, you're increasing a reactant, which will push the reaction in the forward direction and therefore favor the formation of the products.
When a reaction is exothermic, energy is released. Think of it as a product, so if you're increasing temperature, you're increasing a product, which will push the reaction in the reverse direction and therefore favor the formation of the reactants/.
When a reaction is exothermic, energy is released. Think of it as a product, so if you're increasing temperature, you're increasing a product, which will push the reaction in the reverse direction and therefore favor the formation of the reactants/.
-
Eszter Kovacs 1A
- Posts: 100
- Joined: Fri Sep 24, 2021 5:41 am
Re: Exo/Endo reactions
If a reaction is endothermic, increasing the heat favors the forward reaction and after raising the temperature more products will be formed.
I a reaction is exothermic, increasing the temperature will favor the formation of reactants.
I a reaction is exothermic, increasing the temperature will favor the formation of reactants.
Return to “Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions”
Who is online
Users browsing this forum: No registered users and 5 guests

