Problem reads as follows:
A reactor for the production of ammonia by the Haber process is found to be at equilibrium with P N2 3.11 bar, P H2 1.64 bar, and P NH3 23.72 bar. If the partial pressure of N 2 is increased by 1.57 bar, what will be the partial pressure of each gas once equilibrium is re-established?
I found the Kp value to be 41.0 by squaring the NH3 pressure and dividing by PN2*(PH2)^3, however I don't know how to get past the ice table with the quadratic. Any help would be appreciated :)
Textbook Problem 5J.7
Moderators: Chem_Mod, Chem_Admin
-
Brian Nguyen 3B
- Posts: 36
- Joined: Mon Jan 09, 2023 9:57 am
Re: Textbook Problem 5J.7
Hello!
Since you got to the ICE table, you should have gotten the equilibrium concentrations of the reactants and the products: [N2] = 4.68-x, [H2] = 1.64-3x, [NH3] = 23.72+2x. As you have probably found, the chemical equation of this problem is+3H_{2}(g) \rightleftharpoons NH_{3}(g)) . Using this, we can confirm that the Kp for the equation is
. Using this, we can confirm that the Kp for the equation is /([NH_{3}])) . Use your value for Kp, 41.0, and equate it to the equilibrium concentrations in the ICE. this should make the equation,
. Use your value for Kp, 41.0, and equate it to the equilibrium concentrations in the ICE. this should make the equation, ^2}{(4.68-x)(1.64-3x)^{3}}) . Unfortunately, since the equation once solved and equated to 0 is not in the form of
. Unfortunately, since the equation once solved and equated to 0 is not in the form of 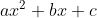 , we can not use the quadratic formula to solve this problem. In addition, the Kp value is too large to be negated. For this problem, you should use a graphing calculator or Desmos to solve for x. Once that is done, the x value calculated should be x = 0.0656. Plugging this into the equilibrium concentrations found in the ICE table, you should get [N2] = 4.61 bars, [H2] = 1.44 bars, [NH3] = 283.85 bars.
, we can not use the quadratic formula to solve this problem. In addition, the Kp value is too large to be negated. For this problem, you should use a graphing calculator or Desmos to solve for x. Once that is done, the x value calculated should be x = 0.0656. Plugging this into the equilibrium concentrations found in the ICE table, you should get [N2] = 4.61 bars, [H2] = 1.44 bars, [NH3] = 283.85 bars.
Since you got to the ICE table, you should have gotten the equilibrium concentrations of the reactants and the products: [N2] = 4.68-x, [H2] = 1.64-3x, [NH3] = 23.72+2x. As you have probably found, the chemical equation of this problem is
Re: Textbook Problem 5J.7
Would we ever be asked a question like this (where the equation is at a higher power than a quadratic) on a midterm? I'm assuming no, since we would not have access to Desmos or a graphing calculator during the midterm right?
Return to “Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions”
Who is online
Users browsing this forum: No registered users and 5 guests

