Thank you!
Textbook Question 6C.17: Justification for Base Strength
Moderators: Chem_Mod, Chem_Admin
-
Sofia Lucido 3L
- Posts: 112
- Joined: Wed Sep 30, 2020 9:51 pm
- Been upvoted: 1 time
Textbook Question 6C.17: Justification for Base Strength
For textbook question 6C.17 it asks: Which is the stronger base, the hypobromite ion BrO- or morphine, 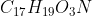 ? I said BrO- was the stronger base because it has a negative charge, but I was wondering if there was additional/stronger reasoning for why BrO- is a stronger base?
? I said BrO- was the stronger base because it has a negative charge, but I was wondering if there was additional/stronger reasoning for why BrO- is a stronger base?
Thank you!
Thank you!
-
reyvalui_3g
- Posts: 100
- Joined: Wed Sep 30, 2020 9:52 pm
Re: Textbook Question 6C.17: Justification for Base Strength
If you compare the pKb values between hypobromite and morphine, you will find that the pKb value of hypobromite is lower than that of morphine. This means that hypobromite is a stronger base.
-
Katherine_Douglas_1F
- Posts: 88
- Joined: Wed Sep 30, 2020 10:01 pm
Re: Textbook Question 6C.17: Justification for Base Strength
If you don't have access to the pKb values, you can use logic to sort of figure out which one is the stronger base. Just as the stronger acids will lose H+ more easily and have more stable anions, stronger bases will gain H+ more easily and have less stable anions. Since BrO- is already negative, it will be more attracted to the H+ ions than C17H19O3N. It is also a less stable anion. All this means that BrO- would be the stronger base.
Return to “Properties & Structures of Inorganic & Organic Acids”
Who is online
Users browsing this forum: No registered users and 7 guests

