How To Tell Which Compounds are Amphoteric?
Moderators: Chem_Mod, Chem_Admin
-
Nancy Li 1C
- Posts: 104
- Joined: Fri Sep 24, 2021 6:46 am
How To Tell Which Compounds are Amphoteric?
Is there an easy way to identify which compounds are amphoteric? Or should we just be memorizing common amphoteric substances?
-
Mona Reddy Kurra 1J
- Posts: 100
- Joined: Fri Sep 24, 2021 5:16 am
- Been upvoted: 1 time
Re: How To Tell Which Compounds are Amphoteric?
Hi! I think one way to determine whether a compound is amphoteric is to see whether it can accept and donate protons, or hydrogen ions. If a compound can do both, then it acts as both an acid and base, so it is amphoteric. For example, 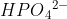 can donate the H+ to become
can donate the H+ to become  or it can accept an H+ to become
or it can accept an H+ to become 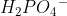 . I hope that helps!
. I hope that helps!
-
AudreyQian1J
- Posts: 110
- Joined: Fri Sep 24, 2021 6:03 am
Re: How To Tell Which Compounds are Amphoteric?
Hello,
One way that could help to identify amphoteric substances is to look for the ability to both add and remove H+ ions from a molecule. In other words, you can look for whether the substance contains more than one H+ ions or multiple charges on its cation or anion. For example, H2PO4- is an amphoteric substance, because removing a hydrogen ion would produce HPO42- + H3O+ and adding a hydrogen ion would produce H3PO4 + OH-. H2PO4- has both hydrogen and a negative charge, producing conjugate acid H3PO4 and conjugate base HPO42-.
Another way is to look at the periodic table, where there is a diagonal band of amphoteric oxides, found in between metal oxides (bases) and nonmetal oxides (acids), that closely matches the diagonal band of metalloids.
One way that could help to identify amphoteric substances is to look for the ability to both add and remove H+ ions from a molecule. In other words, you can look for whether the substance contains more than one H+ ions or multiple charges on its cation or anion. For example, H2PO4- is an amphoteric substance, because removing a hydrogen ion would produce HPO42- + H3O+ and adding a hydrogen ion would produce H3PO4 + OH-. H2PO4- has both hydrogen and a negative charge, producing conjugate acid H3PO4 and conjugate base HPO42-.
Another way is to look at the periodic table, where there is a diagonal band of amphoteric oxides, found in between metal oxides (bases) and nonmetal oxides (acids), that closely matches the diagonal band of metalloids.
Return to “Amphoteric Compounds”
Who is online
Users browsing this forum: No registered users and 6 guests

