Sapling Week 10 #2
Moderators: Chem_Mod, Chem_Admin
-
Joshua Eidam 2A
- Posts: 89
- Joined: Wed Sep 30, 2020 9:58 pm
Sapling Week 10 #2
I am confused as to how I can identify an amphoteric substance from a list of substances. I know that it means it the substance can act both as a Bronsted Acid or Base but how can I identify that just from looking at a list of substances?
-
SainehaMaddineni_3I
- Posts: 117
- Joined: Wed Sep 30, 2020 9:39 pm
- Been upvoted: 1 time
Re: Sapling Week 10 #2
A Bronsted acid can donate H+, so there should be a Hydrogen in the formula. A Bronsted base can accept H+, which can be indicated by a negative charge as it allows the substance to accept an H+. If it meets both descriptions, it can be amphoteric.
-
Sera Aintablian 2E
- Posts: 114
- Joined: Wed Sep 30, 2020 9:35 pm
- Been upvoted: 1 time
Re: Sapling Week 10 #2
If you, for example, consider the molecule HCO3-, it is amphoteric, because it has an H+ proton and a negative charge. As an acid, it produces an H3O+ ion and CO3^2-. This is through donating the H+ proton. As a base, it accepts an H+ and produces H2CO3- and a hydroxide, -OH. Same with HPO4^2-!
-
Jiwon_Chae_3L
- Posts: 99
- Joined: Wed Sep 30, 2020 9:39 pm
Re: Sapling Week 10 #2
An amphoteric substance can gain or lose a hydrogen proton. For a compound to be amphoteric, it either needs hydrogen available in it for it to lose (H2CO3) or a negative charge that can be countered by adding hydrogen (CO3 2-). Going by this rule, something like HSO- is amphoteric.
-
Savannah Torella 1L
- Posts: 52
- Joined: Wed Sep 30, 2020 9:40 pm
Re: Sapling Week 10 #2
As stated above, an amphoteric substance contains hydrogen and a negative charge. The hydrogen allows H+ to be donated, making it a bronsted acid. The negative charge means that a H+ can be accepted, also making it a bronsted base.
-
Manseej Khatri 2B
- Posts: 100
- Joined: Wed Sep 30, 2020 9:42 pm
Re: Sapling Week 10 #2
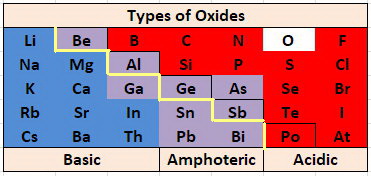
Hi. It might also be good to know the list of metalloid oxides that tend to be amphoteric. This image shows the oxides that tend to be amphoteric.

-
Eve Gross-Sable 1B
- Posts: 102
- Joined: Wed Sep 30, 2020 10:02 pm
- Been upvoted: 1 time
Re: Sapling Week 10 #2
An amphoteric substance must have
- hydrogen present (so it can act as a Bronsted acid to donate H+)
- a feature that allows it to accept an H+ (typically this feature is a negative charge) so it can act as a Bronsted base
So by looking at the formulas and seeing if they fit those bullet points, you should be able to determine which are amphoteric. Hope this helps!
- hydrogen present (so it can act as a Bronsted acid to donate H+)
- a feature that allows it to accept an H+ (typically this feature is a negative charge) so it can act as a Bronsted base
So by looking at the formulas and seeing if they fit those bullet points, you should be able to determine which are amphoteric. Hope this helps!
-
derickngo3d
- Posts: 111
- Joined: Wed Sep 30, 2020 9:51 pm
Return to “Bronsted Acids & Bases”
Who is online
Users browsing this forum: No registered users and 10 guests

