For this question, we are asked to rank the bases (F-, NH3, CH3CO2-, C5H5N) in order of increasing strength.
F- and CH3CO2- are not listed in the table with all the basicity constants, but HF and CH3COOH are listed in the acidity constants table. I solved the problem assuming that the pKb values of F- and CH3CO2- are just their parent base pKa values subtracted from 14, and I got the correct answer. However, I am confused as to why/how this works, since it appears that the pKa and pKb values would be completely different for the weak parent acid/base and its conjugate base/acid.
I may be interpreting this concept incorrectly, so any guidance is appreciated!
6C.9
Moderators: Chem_Mod, Chem_Admin
-
Kaitlin Eblen 1I
- Posts: 104
- Joined: Wed Feb 17, 2021 12:24 am
Re: 6C.9
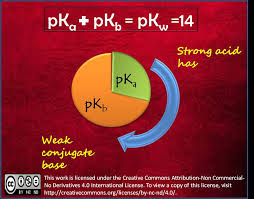
Yes, you did this correctly! That's actually the definition of the Kw = Ka x Kb equation (or in the form 14 = pKa + pKb as you used it). When a pKa value of an acid is plugged into the equation, the resulting pKb value is the basicity constant of the conjugate base. Let me know if that helps!
-
Sadhana Jeyakumar 2J
- Posts: 144
- Joined: Fri Sep 24, 2021 5:25 am
- Been upvoted: 1 time
Re: 6C.9
Kaitlin Eblen 1I wrote:Yes, you did this correctly! That's actually the definition of the Kw = Ka x Kb equation (or in the form 14 = pKa + pKb as you used it). When a pKa value of an acid is plugged into the equation, the resulting pKb value is the basicity constant of the conjugate base. Let me know if that helps!
Yes, your explanation regarding pKa associating with the acid and the resulting pKb value referring to the basicity constant of the conjugate base makes sense. Thank you!
Return to “Acidity & Basicity Constants and The Conjugate Seesaw”
Who is online
Users browsing this forum: No registered users and 6 guests

