Hello,
Below is question 9 on the week 2 homework:
A monoprotic weak acid, HA, is ionized according to the reaction
HA(aq)+H2O(l)↽−−⇀A−(aq)+H3O+(aq) pKa=4.16
where A− is the conjugate base to HA.
For this weak monoprotic acid, the predominant species present at pH 2.46 is
-unknown
-charged
-neutral.
I do not understand what this question is asking me? Can someone please help; I am unsure what I am looking for.
HW 2 Question 9
Moderators: Chem_Mod, Chem_Admin
-
Matthew Bolton 3L
- Posts: 35
- Joined: Mon Jan 09, 2023 10:16 am
Re: HW 2 Question 9
I also didn't initially understand the question. If the 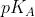 were equal to the pH, then there would be equal concentrations of neutral (protonated) and charged (deprotonated) species. However, because the pH is lower than the
were equal to the pH, then there would be equal concentrations of neutral (protonated) and charged (deprotonated) species. However, because the pH is lower than the 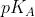 , we know that more protonation will have occurred and that it will be more neutral as a result. I hope this helps.
, we know that more protonation will have occurred and that it will be more neutral as a result. I hope this helps.
-
Rylee Kubo 2K
- Posts: 34
- Joined: Mon Jan 09, 2023 9:30 am
Re: HW 2 Question 9
Hi! At pH values below the pKa, the neutral species of HA will become more predominant in the solution. Since the pH value you were given is below the pKa, the solution becomes more neutral.
-
Abhay Mahajan 3D
- Posts: 42
- Joined: Mon Jan 09, 2023 9:54 am
Re: HW 2 Question 9
Leilani Arcega 2D wrote:Hello,
Below is question 9 on the week 2 homework:
A monoprotic weak acid, HA, is ionized according to the reaction
HA(aq)+H2O(l)↽−−⇀A−(aq)+H3O+(aq) pKa=4.16
where A− is the conjugate base to HA.
For this weak monoprotic acid, the predominant species present at pH 2.46 is
-unknown
-charged
-neutral.
I do not understand what this question is asking me? Can someone please help; I am unsure what I am looking for.
Since the pH < pKa, it means that the solution is more acidic than the acid HA. This means that the acid will not donate its proton to the surrounding solution, leaving it predominantly in the form HA which is not charged (or neutral). This means that you will answer with neutral in the case where pH<pKa.
Return to “Acidity & Basicity Constants and The Conjugate Seesaw”
Who is online
Users browsing this forum: No registered users and 5 guests

