pH to [H+]
Moderators: Chem_Mod, Chem_Admin
-
Ellen Tsai 3D
- Posts: 55
- Joined: Fri Sep 24, 2021 5:55 am
-
Gabby Burgess 2I
- Posts: 101
- Joined: Fri Sep 24, 2021 5:34 am
Re: pH to [H+]
Here are all the formulas I have for switching from pOH to pH, hopefully they help.
pH=-log[H3O+]
[H3O+]=10^(-pH)
pOH=-log[OH-]
[OH-]=10^(-pOH)
pH+pOH=14
[H+]=10^(-pH)
pH=-log[H3O+]
[H3O+]=10^(-pH)
pOH=-log[OH-]
[OH-]=10^(-pOH)
pH+pOH=14
[H+]=10^(-pH)
-
Gianna Sciole 2F
- Posts: 101
- Joined: Fri Sep 24, 2021 7:06 am
Re: pH to [H+]
pH = -log[H+]
because of this, we can also say that:
[H+] = 10^-pH
we also know that:
pOH = -log[OH-] (because [H+]*[OH-]=10^-14).
so we can say that:
[OH-] = 10^-pOH
however if you want it using pH, since we know that pH + pOH =14, it would be: [OH-] = 10^-(14-pH)
because of this, we can also say that:
[H+] = 10^-pH
we also know that:
pOH = -log[OH-] (because [H+]*[OH-]=10^-14).
so we can say that:
[OH-] = 10^-pOH
however if you want it using pH, since we know that pH + pOH =14, it would be: [OH-] = 10^-(14-pH)
-
Diego Gonzalez 1H
- Posts: 50
- Joined: Fri Sep 24, 2021 6:36 am
-
Ameen Shaheen 2I
- Posts: 117
- Joined: Fri Sep 24, 2021 6:45 am
-
Michael 1G
- Posts: 101
- Joined: Fri Sep 24, 2021 6:16 am
- Been upvoted: 1 time
Re: pH to [H+]
The way to calculate the concentration of Hydrogen ion in solution when given the pH is to take 10 and raise it to the -pH power. Ex: if the pH of a solution is 1 the [H+] would be 10^-1=0.1M.
-
Emily Hou 1H
- Posts: 101
- Joined: Fri Sep 24, 2021 7:29 am
-
Sean Sanders 1E
- Posts: 104
- Joined: Fri Sep 24, 2021 5:20 am
- Been upvoted: 1 time
Re: pH to [H+]
To go from pH to [H+] you just need to work backwards. Because we know the formula for pH = -log[H+], we can rearrange the terms to solve for [H+] which gives us [H+]= 10^(-pH)
-
Iman Gauhar 3E
- Posts: 104
- Joined: Fri Sep 24, 2021 6:52 am
Re: pH to [H+]
Hi! Here are the formulas for converting from the different values:
-log[H3O+] = pH
-log[OH-] = pOH
pH + pOH = 14
[pH][pOH] = 10^-14
-log[H3O+] = pH
-log[OH-] = pOH
pH + pOH = 14
[pH][pOH] = 10^-14
-
Joshua Lee 3C
- Posts: 103
- Joined: Fri Sep 24, 2021 6:49 am
Re: pH to [H+]
To find [H+] from the pH equation, you must simply undo the pH equation. pH=-log[H+]. In other words, to rearrange the equation to get [H+], you would undo the base 10 logarithm and get 10^-pH=[H+]. You can do this same process to find [OH-] from the pOH equation.
-
Matthew Nguyen 3G
- Posts: 50
- Joined: Fri Sep 24, 2021 7:18 am
-
Nicole Ton 3C
- Posts: 111
- Joined: Fri Sep 24, 2021 6:51 am
-
Lindsey Walter 3E
- Posts: 103
- Joined: Fri Sep 24, 2021 6:13 am
-
Bela Patel 2B
- Posts: 85
- Joined: Fri Sep 24, 2021 6:40 am
Re: pH to [H+]
To go from pH to [H+], you would do 1*10^-pH and to go from [H+] to pH, you would do -log([H+]).
-
madeleinewright
- Posts: 54
- Joined: Fri Sep 24, 2021 5:36 am
-
Julia Todorov 2F
- Posts: 100
- Joined: Fri Sep 24, 2021 6:03 am
-
Eszter Kovacs 1A
- Posts: 100
- Joined: Fri Sep 24, 2021 5:41 am
-
Hannah Thornton 1F
- Posts: 104
- Joined: Fri Sep 24, 2021 5:53 am
Re: pH to [H+]
The formulas to go back and forth from pH to [H+] are: [H+]=10^(-pH) and pH=-log[H+].
-
Samantha Toscano 2C
- Posts: 106
- Joined: Wed Feb 17, 2021 12:24 am
-
Coraly De Leon
- Posts: 105
- Joined: Fri Sep 24, 2021 5:56 am
-
Anisa Morales 1L
- Posts: 104
- Joined: Fri Sep 24, 2021 7:36 am
-
Madelyn_Rios_2c
- Posts: 110
- Joined: Fri Sep 24, 2021 5:54 am
-
Lizzy Bulla 3K
- Posts: 69
- Joined: Fri Sep 24, 2021 7:20 am
-
Omar Alami 3H
- Posts: 111
- Joined: Fri Sep 24, 2021 7:32 am
Re: pH to [H+]
Hi,
The formula is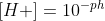 . I find this graphic helpful when trying to remember and figure out which conversions to use depending on the context.
. I find this graphic helpful when trying to remember and figure out which conversions to use depending on the context.
The formula is
- Attachments
-
- download.png (4.19 KiB) Viewed 11250 times
-
Chris Korban 1D
- Posts: 107
- Joined: Fri Sep 24, 2021 6:53 am
-
Sally_Luo_3F
- Posts: 85
- Joined: Fri Sep 24, 2021 5:29 am
-
Omeed Kalan
- Posts: 112
- Joined: Fri Sep 24, 2021 6:10 am
-
Maddie Klee 3K
- Posts: 101
- Joined: Fri Sep 24, 2021 5:59 am
-
Sophia Hartwell 1F
- Posts: 65
- Joined: Fri Sep 24, 2021 5:42 am
Re: pH to [H+]
The formula is [H] = 10^-pH. With that being said, to find [OH], the formula is [OH] = 10^-pOH. Hope this helps!
-
Mahli Martinez 2I
- Posts: 102
- Joined: Fri Sep 24, 2021 7:14 am
Re: pH to [H+]
To convert pH to [H+], use the formula pH= 10^-pH. Some scientific calculators actually show this as the inverse calculation!
-
AndreyCastellanos 3H
- Posts: 59
- Joined: Fri Sep 24, 2021 5:46 am
-
Gabby Burgess 2I
- Posts: 101
- Joined: Fri Sep 24, 2021 5:34 am
Re: pH to [H+]
To pH to [H+], you want to use the formula 10^-pH=[H+]. It also works for the [OH-] to pOH conversion as well.
-
Shannon Clark 1F
- Posts: 112
- Joined: Fri Sep 24, 2021 6:00 am
Re: pH to [H+]
to go from pH to [H+] the equation is:
[H+] = 10^-pH
similarly, to convert from pOH to [OH-]:
[OH-] = 10^-pOH
[H+] = 10^-pH
similarly, to convert from pOH to [OH-]:
[OH-] = 10^-pOH
Re: pH to [H+]
Anything with a p in front of it means take the -log. PH is the -log of the concentration of hydronium ions.
Return to “Calculating pH or pOH for Strong & Weak Acids & Bases”
Who is online
Users browsing this forum: No registered users and 1 guest

