negligible rule [ENDORSED]
Moderators: Chem_Mod, Chem_Admin
-
Jasmine Botello 2F
- Posts: 67
- Joined: Fri Sep 29, 2017 7:04 am
negligible rule
So when K is small enough, then X can be negligible. Does this apply only to weak acids and bases or any type of reaction?
-
Chem_Mod
- Posts: 23858
- Joined: Thu Aug 04, 2011 1:53 pm
- Has upvoted: 1253 times
Re: negligible rule [ENDORSED]
This applies to any equilibrium reaction problem that involves an ICE table process to solve. However, you must also consider your initial concentrations or partial pressures. It is actually the % dissociation of an equilibrium reaction being under 5% which allows you to make the negligible x assumption.
-
Ethan Vuong 3G
- Posts: 51
- Joined: Fri Sep 29, 2017 7:07 am
Re: negligible rule
if we are given an equilibrium constant on a question, is that a good indicator that we are calculating a weak base or acid and therefore eliminate the x?
-
Scott Chin_1E
- Posts: 55
- Joined: Sat Jul 22, 2017 3:00 am
Re: negligible rule
I think first looking at the reactants and products of the problem would be a good indication for whether the problem is a weak acid/base question, especially if they only give you K instead of  or
or 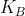 .
.
If they give you or
or 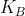 , I think its safe to assume that you are working with weak acids/bases especially since strong acids do not require equilibrium constants because they dissociate fully.
, I think its safe to assume that you are working with weak acids/bases especially since strong acids do not require equilibrium constants because they dissociate fully.
If they give you
Return to “Calculating pH or pOH for Strong & Weak Acids & Bases”
Who is online
Users browsing this forum: No registered users and 11 guests

