NH3 is a weak base ( Kb=1.8×10−5), so the salt NH4Cl acts as a weak acid. What is the pH of a solution that is 0.087
M in NH4Cl at 25 °C?
pH= ???
All I know is that I need to use the Ka*Kb = 10ˆ-14. However, as the hint suggests, I am unsure how to find the Ka for NH4. Do I set up an equilibrium table for that?
I dont even know where to start ;-;
Moderators: Chem_Mod, Chem_Admin
-
Leslie Hernandez 1E
- Posts: 35
- Joined: Mon Jan 09, 2023 2:38 am
-
Irene Oh 2A
- Posts: 56
- Joined: Mon Jan 09, 2023 8:48 am
Re: I dont even know where to start ;-;
Since NH4+ is the conjugate acid of the base NH3, you can find Ka by dividing 10^-14 by the given Kb of NH3. Once you got that, you have to find [H3O+] by using the ICE table to find "X" then do -log(X) to find the pH.
-
Megan Ho 2B
- Posts: 39
- Joined: Mon Jan 09, 2023 8:52 am
Re: I dont even know where to start ;-;
Hello!
To answer your question, we know that the salt NH4Cl dissociates into the ions NH4+ and Cl-. We also know that spectator ions don't affect the pH, so we could disregard the Cl- in this case because its acts as a spectator ion in a reaction with water. NH4+ is the conjugate acid of the weak base NH3, so they can be related through the equation Kw= Ka x Kb. Kb is given for NH3, so to find the Ka for NH4+, you would just need to divide Kw/Kb. Once you have the Ka value for NH4+, you can then proceed with the ICE table for the dissociation of NH4+, as can be represented by the equation NH4+ (aq)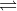 NH3 (aq)+ H+ (aq). After using the equilibrium table to find x, this would be the value of the concentration of [H+] and you could then do -log( [H+] ) to find the pH.
NH3 (aq)+ H+ (aq). After using the equilibrium table to find x, this would be the value of the concentration of [H+] and you could then do -log( [H+] ) to find the pH.
Hope this helps!
To answer your question, we know that the salt NH4Cl dissociates into the ions NH4+ and Cl-. We also know that spectator ions don't affect the pH, so we could disregard the Cl- in this case because its acts as a spectator ion in a reaction with water. NH4+ is the conjugate acid of the weak base NH3, so they can be related through the equation Kw= Ka x Kb. Kb is given for NH3, so to find the Ka for NH4+, you would just need to divide Kw/Kb. Once you have the Ka value for NH4+, you can then proceed with the ICE table for the dissociation of NH4+, as can be represented by the equation NH4+ (aq)
Hope this helps!
-
Simran_Gill_3L
- Posts: 36
- Joined: Mon Jan 09, 2023 10:16 am
Re: I dont even know where to start ;-;
To find the Ka value using the Kb value, use the Kw constant of 1.0*10^-14 or convert to pKa and use pKa+pKb=14 as another way to find the value. I solved this problem by setting up an equation so I can visualize the problem and solving an ICE table to find the concentration of H3O+ and NH4Cl-. Set up a Ka expression with the products and reactants equating the Ka vale you calculated and solving for "x" from the ICE table. H3O+ changes by 'x', so the value you find using a quadratic equation of approximation is the final value for H3O+ concentration. Knowing that pH is the -log of the concentration of H3O+, I found the pH by using -log.
Since pH+pOH=14, If the question were to ask about pOH you'd rearrange this equation to find the pOH value.
Hope this helps!
Since pH+pOH=14, If the question were to ask about pOH you'd rearrange this equation to find the pOH value.
Hope this helps!
Return to “Calculating pH or pOH for Strong & Weak Acids & Bases”
Who is online
Users browsing this forum: No registered users and 4 guests

