Calculating Ka
Moderators: Chem_Mod, Chem_Admin
-
Quinton Sprague 1A
- Posts: 108
- Joined: Wed Sep 30, 2020 9:35 pm
Calculating Ka
In today's lecture, Dr. Lavelle discussed how H2CO3 can actually donate both of its H+ in a reaction with water. In calculating the Ka, he showed how you multiply the products in the numerator and divide this by the solvent. Wondering if actually calculating ourselves, what numbers we put into this equation? Do we use the molarity of products and solvents, or the moles of each? Assuming we will not be calculating this on the final just curious...
Re: Calculating Ka
Ka calculations use concentrations aka molarity, which is indicated by the square brackets [ ] whenever you see like the Ka written like 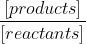 . Also, I believe it's only aqueous compounds from your chemical reaction that are included in Ka.
. Also, I believe it's only aqueous compounds from your chemical reaction that are included in Ka.
-
Rob Tsai 2F
- Posts: 106
- Joined: Wed Sep 30, 2020 10:09 pm
Re: Calculating Ka
In this example, you would divide the product of the concentrations of the products over the concentration of the solvent:
AH <—> A- (aq) + H+ (aq) leads to Ka =( [A-][H+] )/[AH]
Concentrations, if they aren't given, would be calculated through our molarity formula M = n/V, where n=moles and v= volume in liters.
AH <—> A- (aq) + H+ (aq) leads to Ka =( [A-][H+] )/[AH]
Concentrations, if they aren't given, would be calculated through our molarity formula M = n/V, where n=moles and v= volume in liters.
-
Michael Sun Dis 3G
- Posts: 50
- Joined: Wed Sep 30, 2020 9:37 pm
Re: Calculating Ka
Since Ka requires the concentrations of relevant reactants and products, you would need to use molarity.
-
Joshua Eidam 2A
- Posts: 89
- Joined: Wed Sep 30, 2020 9:58 pm
Re: Calculating Ka
So what is the exact equation for Ka? Also if only aqueous samples are used in calculating the Ka, then are any samples in different forms (solid, liquid, gas) just disregarded?
-
Jordi M 2I
- Posts: 152
- Joined: Wed Sep 30, 2020 9:40 pm
Re: Calculating Ka
Rob Tsai 2F wrote:In this example, you would divide the product of the concentrations of the products over the concentration of the solvent:
AH <—> A- (aq) + H+ (aq) leads to Ka =( [A-][H+] )/[AH]
Concentrations, if they aren't given, would be calculated through our molarity formula M = n/V, where n=moles and v= volume in liters.
Wouldn't the solvent be water? I think you mean concentration of solute since we never include water in the Ka calculation.
-
Gigi Elizarraras 2C
- Posts: 100
- Joined: Wed Sep 30, 2020 9:41 pm
Re: Calculating Ka
You would use the molarities of the products and the reactants so to calculate KA you need to know the concentrations:)
-
Lorraine Jiang 2C
- Posts: 112
- Joined: Wed Sep 30, 2020 9:54 pm
- Been upvoted: 1 time
Re: Calculating Ka
Hi! The formula for Ka will be [A-][H3O+]/[HA] so that is the concentration of the anion multiplies the concentration of the H+ ion divided by the concentration of the acid.
Hope it helps!
Hope it helps!
-
Rohit Srinivas 2D
- Posts: 101
- Joined: Wed Sep 30, 2020 9:52 pm
Re: Calculating Ka
We add in molarity (concentration). We haven't done kinetics yet but whenever we see the [] that indicates concentration.
Return to “Polyprotic Acids & Bases”
Who is online
Users browsing this forum: No registered users and 8 guests

