polyprotic acids
Moderators: Chem_Mod, Chem_Admin
-
Jayza Calderon 2A
- Posts: 57
- Joined: Fri Sep 24, 2021 6:43 am
-
Brandon Yu
- Posts: 121
- Joined: Fri Sep 24, 2021 6:07 am
Re: polyprotic acids
Polyprotic acids are specific acids that are capable of losing more than a single proton per molecule in acid-base reactions. (In other words, acids that have more than one ionizable H+ atom per molecule).
-
Ethan Wang 1L
- Posts: 104
- Joined: Fri Sep 24, 2021 6:34 am
Re: polyprotic acids
The easiest way is to see if the molecule 1) is an acid and 2) has more than one hydrogen atom. More often than not, an acid with multiple hydrogen atoms will ultimately be capable of losing multiple hydrogen atoms.
-
Alex Yeghikian 1C
- Posts: 104
- Joined: Fri Sep 24, 2021 6:27 am
Re: polyprotic acids
Some common polyprotic acids are sulfuric acid (H2SO4), phosphoric acid (H3PO4), and carbonic acid (H2CO3). In general, multiple hydrogens in the acid indicate polyproticity, since the acid can then deprotonate more than once. As an example, sulfuric acid is diprotic:
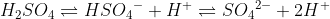
Return to “Polyprotic Acids & Bases”
Who is online
Users browsing this forum: No registered users and 6 guests

