During the analysis of an unknown acid HA, a 0.010 m solution of the sodium salt of the acid was found to have a pH of 10.35. Use Table 12.1 to write the formula of the acid.
I don't clearly understand the answer key, please help.
Thanks!!
#12.75
Moderators: Chem_Mod, Chem_Admin
-
Yiling Liu 1N
- Posts: 25
- Joined: Sat Jul 09, 2016 3:00 am
Re: #12.75
I'm having difficulty solving this problem as well.
The fact that there is a sodium salt of an unknown acid with pH 10.35 seems inherently contradictory to me -- wouldn't that mean it is a base, since the pH is >7?
I also don't understand how in the answer key Kb was solved with 0.01 in the denominator instead of what one might expect from the previous problems, which is to put something like "0.01-x" in this case in the denominator. Because when setting up the ICE charts, doesn't the original concentration of the substance decrease by x amount in order for the products to increase by x amount?
The fact that there is a sodium salt of an unknown acid with pH 10.35 seems inherently contradictory to me -- wouldn't that mean it is a base, since the pH is >7?
I also don't understand how in the answer key Kb was solved with 0.01 in the denominator instead of what one might expect from the previous problems, which is to put something like "0.01-x" in this case in the denominator. Because when setting up the ICE charts, doesn't the original concentration of the substance decrease by x amount in order for the products to increase by x amount?
-
Joseph Nguyen 3L
- Posts: 24
- Joined: Fri Jul 22, 2016 3:00 am
Re: #12.75
Having a sodium salt of the acid means you have .010M of Na(A). Thus the dissociation would be .010M Na+ and A-, where Na+ does not affect the pH.
A- + H2O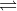 HA + OH-
HA + OH-
Using the ICE chart, you would get
A- HA OH-
I .010M 0 0
C -x +x +x
E .010 - x x x
Kb = [OH-][HA] / [A-] = x^2/(.010-x)
Ka = Kw/Kb = [H30+][A-] / [HA]
Knowing that the pH is 10.35, you can take the antilog (10^-10.35) to find the concentration of H30+ ions.
Because [OH-] = x, [H3O+] = (1 * 10^-14) / x. With that, you can solve for x.
Plug back x into the Ka equation, and you check it with the Ka values at Table 12.1, and you will see it matches HBrO's Ka value.
A- + H2O
Using the ICE chart, you would get
A- HA OH-
I .010M 0 0
C -x +x +x
E .010 - x x x
Kb = [OH-][HA] / [A-] = x^2/(.010-x)
Ka = Kw/Kb = [H30+][A-] / [HA]
Knowing that the pH is 10.35, you can take the antilog (10^-10.35) to find the concentration of H30+ ions.
Because [OH-] = x, [H3O+] = (1 * 10^-14) / x. With that, you can solve for x.
Plug back x into the Ka equation, and you check it with the Ka values at Table 12.1, and you will see it matches HBrO's Ka value.
-
Joseph Nguyen 3L
- Posts: 24
- Joined: Fri Jul 22, 2016 3:00 am
Re: #12.75
Having a sodium salt of the acid means you have .010M of Na(A). Thus the dissociation would be .010M Na+ and A-, where Na+ does not affect the pH.
A- + H2O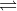 HA + OH-
HA + OH-
Using the ICE chart, you would get
A- HA OH-
I .010M 0 0
C -x +x +x
E .010 - x x x
Kb = [OH-][HA] / [A-] = x^2/(.010-x)
Ka = Kw/Kb = [H30+][A-] / [HA]
Knowing that the pH is 10.35, you can take the antilog (10^-10.35) to find the concentration of H30+ ions.
Because [OH-] = x, [H3O+] = (1 * 10^-14) / x. With that, you can solve for x.
Plug back x into the Ka equation, and you check it with the Ka values at Table 12.1, and you will see it matches HBrO's Ka value.
A- + H2O
Using the ICE chart, you would get
A- HA OH-
I .010M 0 0
C -x +x +x
E .010 - x x x
Kb = [OH-][HA] / [A-] = x^2/(.010-x)
Ka = Kw/Kb = [H30+][A-] / [HA]
Knowing that the pH is 10.35, you can take the antilog (10^-10.35) to find the concentration of H30+ ions.
Because [OH-] = x, [H3O+] = (1 * 10^-14) / x. With that, you can solve for x.
Plug back x into the Ka equation, and you check it with the Ka values at Table 12.1, and you will see it matches HBrO's Ka value.
Return to “Identifying Acidic & Basic Salts”
Who is online
Users browsing this forum: No registered users and 4 guests

