Identifying Acids and Bases
Moderators: Chem_Mod, Chem_Admin
-
Alexandru Georgescu 3A
- Posts: 34
- Joined: Mon Jan 09, 2023 9:41 am
Identifying Acids and Bases
I am struggling in writing the equations for acids and bases because I am not sure how to identify these substances. How can you tell if a substance is an acid or base.
-
Alexa Le 3F
- Posts: 42
- Joined: Mon Jan 09, 2023 10:01 am
Re: Identifying Acids and Bases
The easiest way to tell is usually by just looking at the chemical formula. If you look at enough of them, you can generally start to see a pattern of general similarities for chemical formulas for acids and bases. If the molecule has a lot of H+ ions, then it's probably an acid. However, if you see OH- in the formula, it's probably a base. For example, if you have the chemical formula Na(OH)2, you can see the OH that indicates it's a base. If that also doesn't work, you can add H2O to see what happens. Hope that helps!
-
Andreea Soricut 1F
- Posts: 34
- Joined: Fri Sep 24, 2021 5:55 am
Re: Identifying Acids and Bases
One strategy would be to count the number of hydrogens present in each of the substances before and after the reaction. If the number of hydrogens decreased from the reactants to the products side, then that substance (on the reactants side) will be an acid since it donated hydrogen ions. If the number of hydrogens increased from the reactants to the products side, then that substance (on the reactants side) will be a base since it accepted hydrogen ions.
Another helpful strategy would be to memorize the list of strong and weak acids and bases so that you can quickly identify the acids and bases from the start of solving the problem.
Another helpful strategy would be to memorize the list of strong and weak acids and bases so that you can quickly identify the acids and bases from the start of solving the problem.
-
Ellie Garcia 2L
- Posts: 18
- Joined: Mon Jan 09, 2023 9:35 am
Re: Identifying Acids and Bases
Hi Alexandru!
I like to think of acid as the donators and bases as the recievers of what was given up from an acid. Let's use the equation: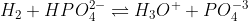
When we look at the equation, we can see that HPO4 (-2 charge) will be giving up it's hydrogen to H2O. Due to this, H2O becomes H3O (with a positive charge) and HPO4 (-2 charge) becomes PO4 (with a -3 charge since it lost a positive charge). Here it's clear to see with one is an acid and which is a base. However, if you wanted to identify the conjugate base and acid, you'd just do the reverse in this case. PO4 (-3 charge) would end up being the conjugate base and H3O (+ charge) would end up being the conjugate acid (since it's giving up a H). Hope this explanation helps and happy studying! ^^
I like to think of acid as the donators and bases as the recievers of what was given up from an acid. Let's use the equation:
When we look at the equation, we can see that HPO4 (-2 charge) will be giving up it's hydrogen to H2O. Due to this, H2O becomes H3O (with a positive charge) and HPO4 (-2 charge) becomes PO4 (with a -3 charge since it lost a positive charge). Here it's clear to see with one is an acid and which is a base. However, if you wanted to identify the conjugate base and acid, you'd just do the reverse in this case. PO4 (-3 charge) would end up being the conjugate base and H3O (+ charge) would end up being the conjugate acid (since it's giving up a H). Hope this explanation helps and happy studying! ^^
Return to “Identifying Acidic & Basic Salts”
Who is online
Users browsing this forum: No registered users and 1 guest

