-OH vs OH-
Moderators: Chem_Mod, Chem_Admin
-
Anya Holbrook 1E
- Posts: 104
- Joined: Wed Sep 30, 2020 9:38 pm
-OH vs OH-
This doesn't really have anything to do with calculating pH, but why is it that sometimes in Dr. Lavelle's notes he put -OH instead of OH-? Don't charges on a molecule or ion go after the naming?
-
Evie Li_1H
- Posts: 105
- Joined: Wed Sep 30, 2020 9:48 pm
- Been upvoted: 1 time
Re: -OH vs OH-
I don't think it's that big of a deal whichever side you decide to put the charge on but, traditionally, the charge will come after the ion.
-
IshanModiDis2L
- Posts: 106
- Joined: Wed Sep 30, 2020 9:49 pm
Re: -OH vs OH-
I believe the charge usually will come after the ion but I do not think it rly does matter too much. Only time I would really consider it is when drawing a structure.
-
JaylinWangDis1L
- Posts: 102
- Joined: Wed Sep 30, 2020 10:09 pm
Re: -OH vs OH-
I was also a little confused on this at first but I think the placement of the negative sign doesn't really matter. It's usually written after the ion.
-
Cooper_Geralds_3B
- Posts: 100
- Joined: Wed Sep 30, 2020 10:07 pm
Re: -OH vs OH-
Hi, I noticed the -OH in today's lecture as well and am used to seeing OH- in both his lectures and the homework, but I am pretty certain that they are the same!
-
Ryan_Page_1J
- Posts: 100
- Joined: Wed Sep 30, 2020 10:03 pm
- Been upvoted: 1 time
Re: -OH vs OH-
It does not really matter. When he puts the - before like -OH, he is trying to emphasize that the oxygen is the atom with the negative charge and not the hydrogen.
I hope this helps!
I hope this helps!
-
Brian Bui 3H
- Posts: 101
- Joined: Wed Sep 30, 2020 9:42 pm
Re: -OH vs OH-
Does not matter, but I think that he puts the negative charge on the O because that O has a formal charge of -1, which is typically indicated with just a negative sign.
-
SamayaJoshi1A
- Posts: 104
- Joined: Wed Sep 30, 2020 9:37 pm
-
Isabel_Eslabon_2G
- Posts: 108
- Joined: Wed Sep 30, 2020 9:50 pm
- Been upvoted: 1 time
Re: -OH vs OH-
It's the same thing. In general, 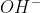 is how you'd normally write it when writing a chemical equation.
is how you'd normally write it when writing a chemical equation. 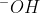 is used more when writing diagrams or mechanisms to emphasize that the negative charge is from the oxygen, not the hydrogen.
is used more when writing diagrams or mechanisms to emphasize that the negative charge is from the oxygen, not the hydrogen.
-
Linette Choi 3L
- Posts: 103
- Joined: Wed Sep 30, 2020 9:51 pm
-
Katelynn Shaheen 2C
- Posts: 51
- Joined: Tue Nov 05, 2019 12:18 am
-
August Blum Dis 3D
- Posts: 54
- Joined: Fri Sep 24, 2021 5:30 am
Re: -OH vs OH-
Sometimes the notation is different for OH-, but either way, the molecule is the same. It shouldn't really matter how it is written. However, some put the charge next to the oxygen to demonstrate that it is the oxygen with the negative charge.
-
Sarah Lesmeister 2F
- Posts: 50
- Joined: Fri Sep 24, 2021 5:14 am
-
Anton Truskovsky 2K
- Posts: 50
- Joined: Fri Sep 24, 2021 7:03 am
Re: -OH vs OH-
They mean the same thing. Use usually would use -OH in like a diagram to signify that it's definitely the oxygen that carries a negative charge.
-
trevina_brown_2A
- Posts: 105
- Joined: Wed Feb 03, 2021 12:15 am
-
Alessandra Marotta 3L
- Posts: 50
- Joined: Fri Sep 24, 2021 5:17 am
Re: -OH vs OH-
I think it is most commonly written as OH-, but both -OH and OH- mean the same thing!
Re: -OH vs OH-
as everyone is saying, it is the same thing, but I would recommend writing it as OH-. Hope this helps!
-
SuryaDham 3E
- Posts: 100
- Joined: Fri Sep 24, 2021 7:29 am
-
N Kanuri 2E
- Posts: 109
- Joined: Fri Sep 24, 2021 5:41 am
- Been upvoted: 1 time
Re: -OH vs OH-
As previous comments have said, I don't believe it matters. I think it is conventionally written as OH-, so that is what I use.
Re: -OH vs OH-
hello, I was a little confused on this at first but I think the placement of the negative sign doesn't really matter.
-
Kristen Bansil 1G
- Posts: 105
- Joined: Fri Sep 24, 2021 7:18 am
Re: -OH vs OH-
Hey there! I believe they are both the same but I usually keep the negative sign next to the O to remind me of its electronegativity, hope this helped!
-
Vivek Chotai 2C
- Posts: 138
- Joined: Fri Sep 24, 2021 7:25 am
- Been upvoted: 1 time
Re: -OH vs OH-
OH- might be referring to the charge, but -OH might be representing the covalent bond between O and the other atom.
-
Acharya Ranawat 3E
- Posts: 102
- Joined: Fri Sep 24, 2021 6:39 am
Re: -OH vs OH-
I believe that these represent the same thing. The only difference may be that the -OH represents the bond between atoms, and the OH- may just represent the charge of it. Hope this helps!
-
Reece Fong 2k
- Posts: 105
- Joined: Fri Sep 24, 2021 6:21 am
Re: -OH vs OH-
Usually it just shows that the negative end of the dipole is by the O. Also though, OH is a connecting group sometimes and can be denoted as -OH (- being the side connecting).
-
Alex Luong 3H
- Posts: 108
- Joined: Fri Sep 24, 2021 7:18 am
Re: -OH vs OH-
It shows which part of the molecule is slightly negative, which helps some see which part of the OH- molecule is binding to the entire molecule.
-
Srikar_Chintala_1E
- Posts: 105
- Joined: Fri Sep 24, 2021 6:33 am
Re: -OH vs OH-
I don't think it holds much of a difference. It is probably just personal preference.
-
daniellediem1k
- Posts: 109
- Joined: Fri Sep 24, 2021 6:00 am
-
Katherine Li 1A
- Posts: 110
- Joined: Fri Sep 24, 2021 6:38 am
Re: -OH vs OH-
The hydroxyl ion is written as OH-. -OH refers to the hydroxyl group, where -OH is attached to some other molecule. The dash is only a minus sign in the first example.
-
Jiayin Yola Yan 1G
- Posts: 60
- Joined: Fri Sep 24, 2021 6:20 am
-
Talia Tam 3L
- Posts: 107
- Joined: Fri Sep 24, 2021 5:38 am
-
Carla Bruebach 1C
- Posts: 100
- Joined: Fri Sep 24, 2021 5:54 am
-
Claire Kim 1F
- Posts: 102
- Joined: Fri Sep 24, 2021 6:27 am
Re: -OH vs OH-
Anya Holbrook 1E wrote:This doesn't really have anything to do with calculating pH, but why is it that sometimes in Dr. Lavelle's notes he put -OH instead of OH-? Don't charges on a molecule or ion go after the naming?
I think it's usually presented as OH- but both should mean the same thing.
Return to “Calculating the pH of Salt Solutions”
Who is online
Users browsing this forum: No registered users and 3 guests

