The problem is attached as an image.
Can someone explain the whole problem? When do we need to use an ice table to answer the question "what's an initial pH" and when can we just use pH= -log (H+) directly, as in question 13.27? Also, why does the solutions guide use the equation CH3COOH+H2O-->H3O+ + CH3CO2- in part a) but H2O+ CH3CO2- --> CH3COOH + OH- in part f) ?
13.35 Titration of CH3COOH with NaOH
Moderators: Chem_Mod, Chem_Admin
-
Alison Ou 14a
- Posts: 32
- Joined: Fri Sep 25, 2015 3:00 am
-
Chem_Mod
- Posts: 23858
- Joined: Thu Aug 04, 2011 1:53 pm
- Has upvoted: 1253 times
Re: 13.35 Titration of CH3COOH with NaOH
To start, you use an ice box to solve for initial pH if the initial equilibrium concentrations are unknown. Basically, if you just created an acid solution by dissolving weak acid in water, you can only calculate the pH after the acid has reached equilibrium in its new solution by breaking into conjugate base and H+. This is not the case for strong acids, such as HCL in 13.27, since they always completely dissociate into conjugate base and H+; however, for weak acids, this must be done since weak acids vary in how much they deprotonate. After you have found the equilibrium [H+] by doing ICE box, you can use the pH = -log[H+] to find the pH value. If the question tells you [H+] at equilibrium, then you can go ahead and calculate pH with ICE box since you already know the equilibrium concentration.
Since this is a titration question, you are basically adding base to react with your acid solution in order to change the overall pH of the solution in order to figure out how much base is needed to neutralize the acid. For every mole (mol, not M; this is a very important distinction since you are changing the volume every time you add base solution) of base you add, you eliminate one mole of weak acid by turning it into conjugate base. This means that, at the stoichiometric point, all of your weak acid (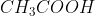 ) has been converted into its conjugate base (
) has been converted into its conjugate base (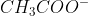 ). All you have in your stoichiometric point solution is conjugate base, so to reach a new equilibrium, this conjugate base must protonate to return to its weak acid form. This is the reason for the different reaction in part f), which is in fact the base equilibrium equation (you use
). All you have in your stoichiometric point solution is conjugate base, so to reach a new equilibrium, this conjugate base must protonate to return to its weak acid form. This is the reason for the different reaction in part f), which is in fact the base equilibrium equation (you use 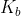 now).
now).
If you want to think of it as a general rule, you will always switch from using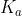 to
to 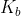 or vice versa depending on what you started with, and the equation will switch so that your reactant will now be the conjugate substance. If you are titrating weak acid, you will use
or vice versa depending on what you started with, and the equation will switch so that your reactant will now be the conjugate substance. If you are titrating weak acid, you will use 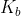 to find concentrations at the stoichiometric point, and if you are titrating a weak base, you will use
to find concentrations at the stoichiometric point, and if you are titrating a weak base, you will use 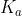 to find concentrations at the stoichiometric point.
to find concentrations at the stoichiometric point.
Summary: In this case, we started with the weak acid (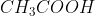 ), so we start with
), so we start with 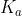 . At stoichiometric point, we now only have conjugate base
. At stoichiometric point, we now only have conjugate base 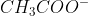 , but we want to reach equilibrium again, so we ICE box again but use the new equation in f) and
, but we want to reach equilibrium again, so we ICE box again but use the new equation in f) and 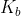 to find our new concentrations and, with some manipulation, our pH.
to find our new concentrations and, with some manipulation, our pH.
Now, go give it a try! Sorry it is lengthy, but I hope this helps.
Since this is a titration question, you are basically adding base to react with your acid solution in order to change the overall pH of the solution in order to figure out how much base is needed to neutralize the acid. For every mole (mol, not M; this is a very important distinction since you are changing the volume every time you add base solution) of base you add, you eliminate one mole of weak acid by turning it into conjugate base. This means that, at the stoichiometric point, all of your weak acid (
If you want to think of it as a general rule, you will always switch from using
Summary: In this case, we started with the weak acid (
Now, go give it a try! Sorry it is lengthy, but I hope this helps.
-
Alison Ou 14a
- Posts: 32
- Joined: Fri Sep 25, 2015 3:00 am
Return to “*Titrations & Titration Calculations”
Who is online
Users browsing this forum: No registered users and 4 guests

