Here's Question 8.21:
A piece of copper of mass 20.0 g at 100.0 degrees Celsius is placed in a
vessel of negligible heat capacity but containing 50.7 g of water
at 22.0 degrees Celsius. Calculate the final temperature of the water. Assume
that no energy is lost to the surroundings.
I understand how the solution manual solves it. However, I was wondering if there is an easier method to solve this type of question instead of using equations that might take longer. Please let me know when possible. Thank you.
Question on 8.21
Moderators: Chem_Mod, Chem_Admin
-
Chem_Mod
- Posts: 23858
- Joined: Thu Aug 04, 2011 1:53 pm
- Has upvoted: 1253 times
Re: Question on 8.21
Using the 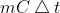 equation is good practice for students to problem solve. It exemplifies the theory of thermodynamics and must be quantified through use of equation.
equation is good practice for students to problem solve. It exemplifies the theory of thermodynamics and must be quantified through use of equation.
-
Sohini Halder 1G
- Posts: 58
- Joined: Thu Jul 13, 2017 3:00 am
- Been upvoted: 1 time
Re: Question on 8.21
Essentially, -q(copper)=q(water), which is saying that the heat given off by the copper is the heat absorbed by water. You have to use the q=m(delta)T equation to solve given the information you have. I set the equations to each other and solve.
-
AtreyiMitra2L
- Posts: 169
- Joined: Fri Sep 29, 2017 7:03 am
- Been upvoted: 1 time
Re: Question on 8.21
In this question, you have to understand that the heat released by one will be absorbed by the other. Consequently, you will use -q(copper) = q(water). The initial temp of copper is 100 C and the initial temp of water 22 C. Because you know the masses and can find the specific heat, you should automatically know that you will have to use the equations. In this course, I believe Dr. Lavelle wants us to only focus on using the equations.
Return to “Phase Changes & Related Calculations”
Who is online
Users browsing this forum: No registered users and 9 guests

