Calculating the Initial Temperature Of An Object
Moderators: Chem_Mod, Chem_Admin
-
Jesus Rodriguez 1J
- Posts: 53
- Joined: Sat Jul 22, 2017 3:00 am
Calculating the Initial Temperature Of An Object
A 248 g piece of copper is dropped into 390ml of water at 22.6C. The final temperature of the water is 39.9C. Can someone explain how we would calculate the initial temperature of the copper piece?
-
Janet Nguyen 2H
- Posts: 51
- Joined: Fri Sep 29, 2017 7:05 am
Re: Calculating the Initial Temperature Of An Object
You would find the q of the water using the change in temperature and q=mC(deltaT). Then set the negative that equal to the amount of heat the copper lost (they should give you the specific heat capacity of copper) and solve for delta T. Use delta T to then find initial temperature. (the final temperature of the copper and water should be the same)
-
RussellChin_3A
- Posts: 42
- Joined: Sat Jul 22, 2017 3:01 am
Re: Calculating the Initial Temperature Of An Object
use q=mC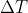
and plug the respective values into this:
-Qmetal=Qwater
and plug the respective values into this:
-Qmetal=Qwater
-
Silvino Jimenez 1A
- Posts: 52
- Joined: Fri Sep 29, 2017 7:04 am
Re: Calculating the Initial Temperature Of An Object
The answer I'm getting is approx:
40.2 C for initial Temp. of Cu
This makes sense b/c in order for the water to increase temp, it had to transfer from a hotter object (2nd Law of Thermo)
40.2 C for initial Temp. of Cu
This makes sense b/c in order for the water to increase temp, it had to transfer from a hotter object (2nd Law of Thermo)
Return to “Phase Changes & Related Calculations”
Who is online
Users browsing this forum: No registered users and 16 guests

